
Concept explainers
(a)
Interpretation: To indicate whether
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
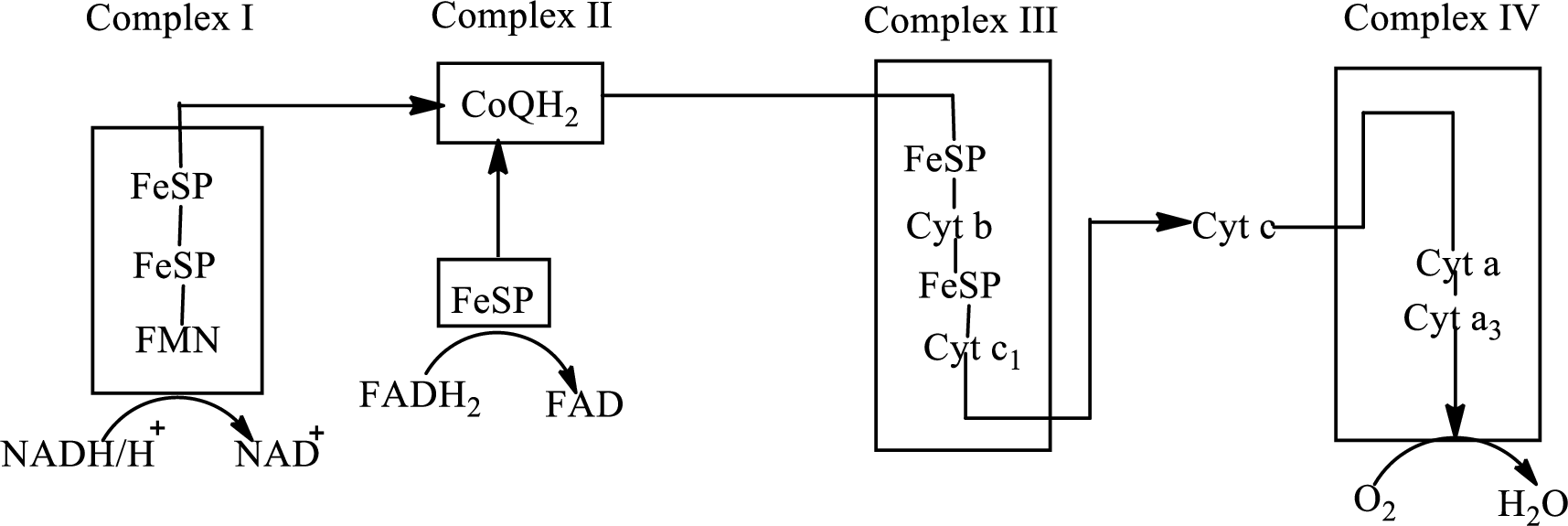
Redox
Here A is oxidized form and AH is reduced form.
(a)
Answer to Problem 12.87EP
Explanation of Solution
(b)
Interpretation: To indicate whether
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
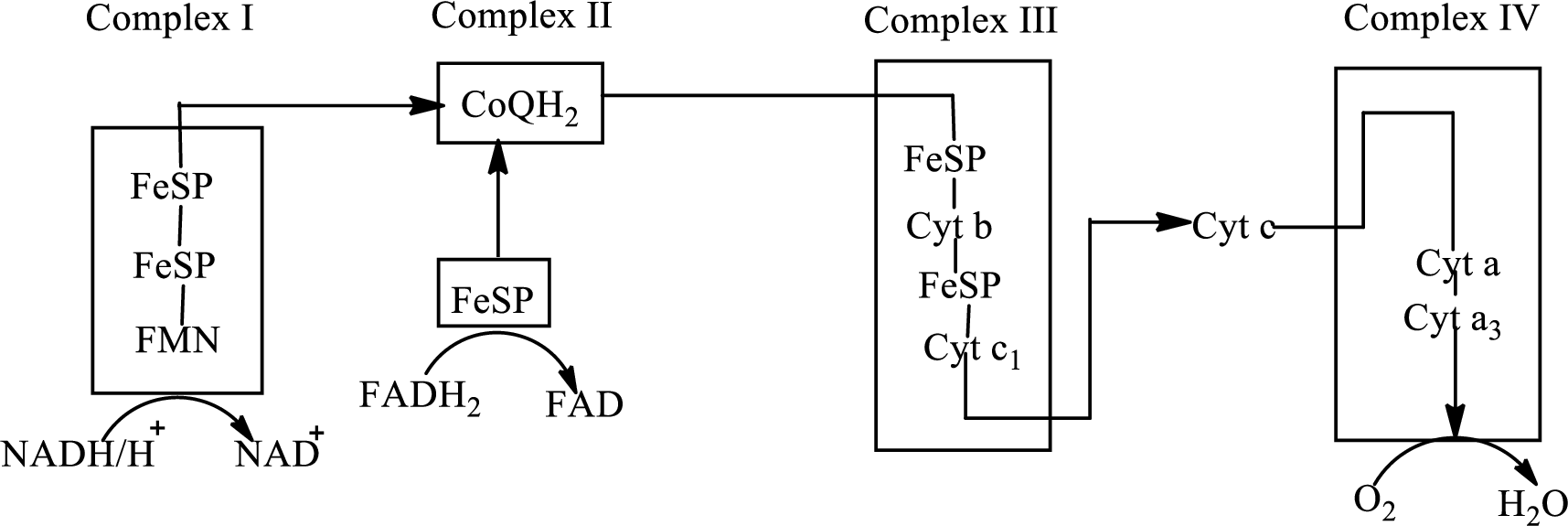
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is
Here A is oxidized form and AH is reduced form.
(b)
Answer to Problem 12.87EP
Explanation of Solution
(c)
Interpretation: To indicate whether
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
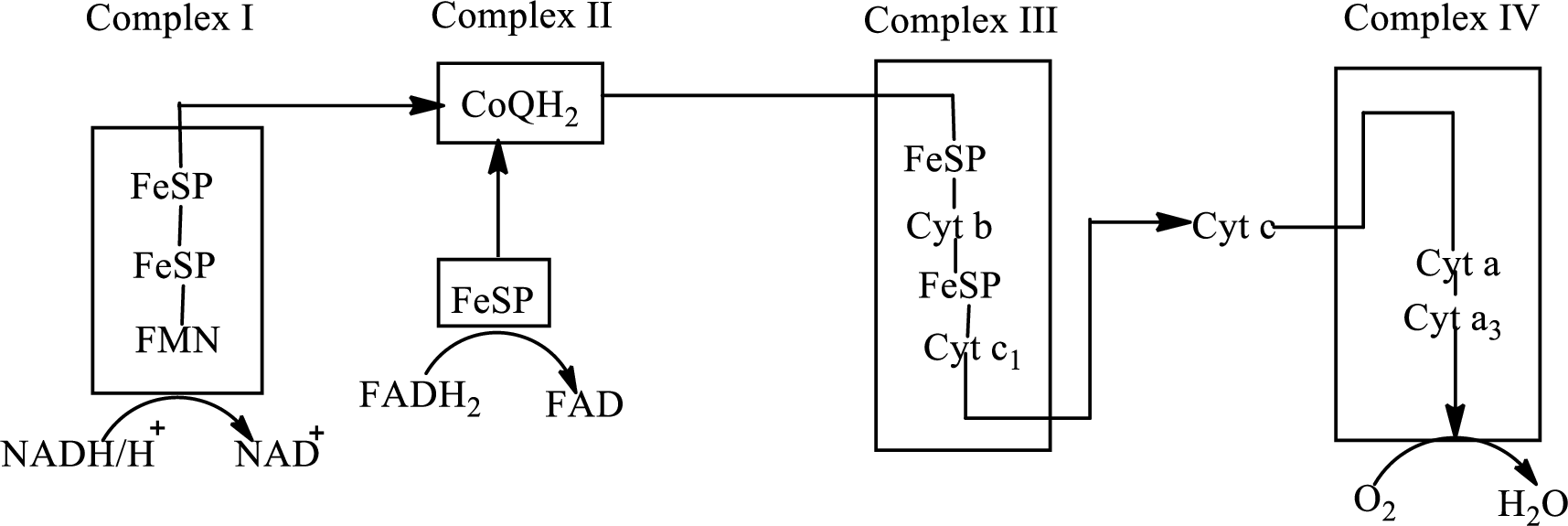
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is
Here A is oxidized form and AH is reduced form.
(c)
Answer to Problem 12.87EP
NADH is the reduced form of the nicotinamide adenine dinucleotide.
Explanation of Solution
In complex I, electrons are transferred from the
The reaction of the oxidation of
Here,
(d)
Interpretation: To indicate whether is in its oxidized form or its reduced form.
Concept introduction: Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
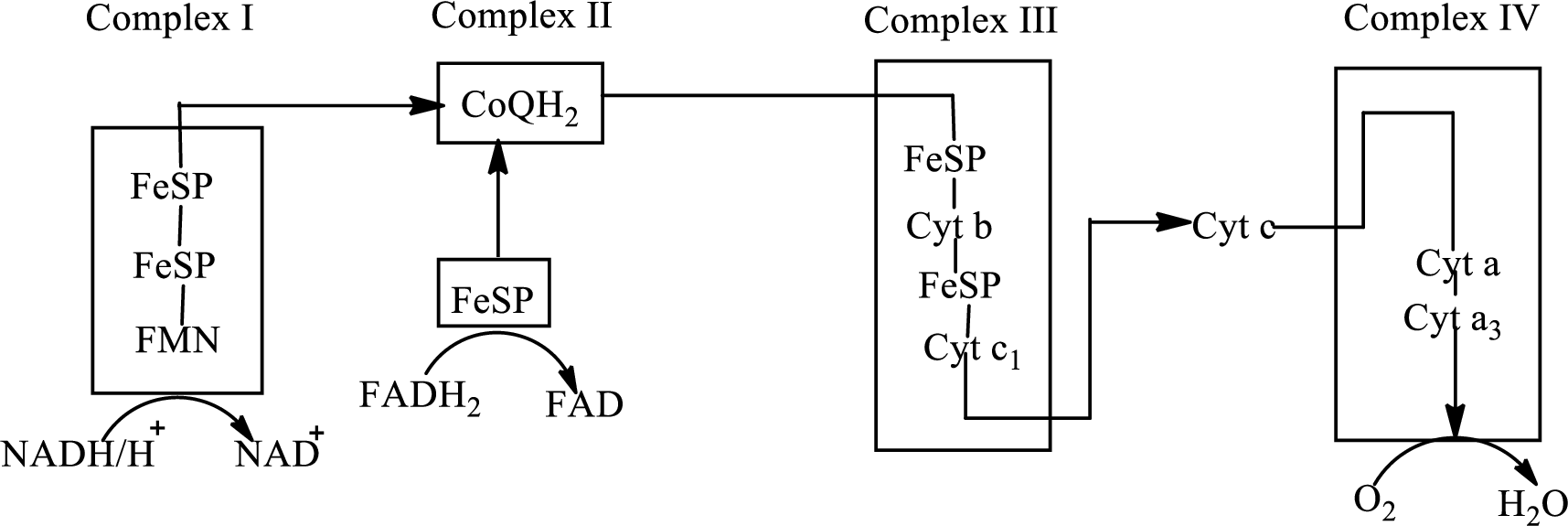
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of redox reaction is
Here A is oxidized form and AH is reduced form.
(d)
Answer to Problem 12.87EP
FAD is the oxidized form of the flavin adenine dinucleotide.
Explanation of Solution
In the complex II, electrons are transferred from the
Here,
Want to see more full solutions like this?
Chapter 12 Solutions
Organic And Biological Chemistry
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



