
Interpretation:
The
Concept introduction:
The group of symmetry operations of which atleast one point is kept fixed is called point group. The symmetry operations can be identity, rotation, reflection, inversion and improper rotation.
Answer to Problem 13.43E
The
The coordinates for hydrogen in
Explanation of Solution
The structure of ammonia molecule is,
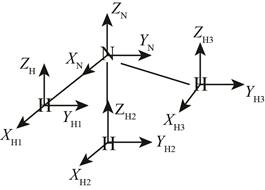
Figure 1
The total coordinates in the given system is
The ammonia molecule has non-linear structure and
On applying the
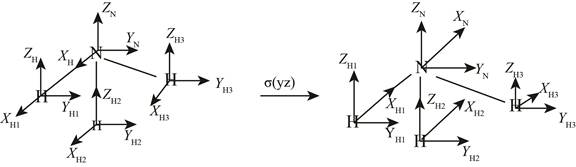
Figure 2
The
Similarly on applying the
The
Similarly on applying the
The
The coordinates for hydrogen for
The
The coordinates for hydrogen in
Want to see more full solutions like this?
Chapter 13 Solutions
Physical Chemistry
- Construct the Hckel determinants for cyclobutadiene and cyclopentadiene. In what ways are they alike? In what ways are they different?arrow_forwardAssume that you are evaluating the integral of products of functions having symmetry labels in exercise 13.60, parts a-d. Which integrals, if any, are exactly zero due to symmetry considerations?arrow_forwardIdentify the symmetry elements present in the following objects. a The Eiffel Tower. You may have to look up a picture of it if you dont remember its shape b Any book ignore the printing. c An octagonal wood block. d A jack from the set of jacks pictured here: Note that some of the points end differently.arrow_forward
- 4. Show that the function x y z has symmetry species A1 in pt group D2,arrow_forwardWhat is the symmetry element corresponding to (a) Cn, (b) s, (c) i, (d) Sn? What is the symmetry operation corresponding to ?53?arrow_forwardDetermine whether the integral over x and y is necessarily zero in a molecule with symmetry D2 by using its symmetry properties.arrow_forward
- 1.Consider an XeOCl4 molecule. a) Determine the point group of the molecule. b) Determine a hybridization of the Xe atom. c) Determine a symmetry of the vibrational modes of the molecule. Please answer completelyarrow_forwardConsider phosphorus pentafluoride, PF5, D3h symmetry. The reducible representation for the 18 degrees of freedom is: A'1= 2 A'2= 1 E'= 4 A''1=0 A''2=3 E''=2 and given.. E=? 2C3(z)= 0 3C'2= -2 σ h(xy)=4 2S3= -2 σ v(yz)=4 what is E? 1.) What are the symmetry labels for the rotational modes? 2.) What are the symmetry labels for the translational modes? 3.) What are the symmetry labels for the vibrational modes? 4.) How many vibrational modes are present in this molecule? 5.) Which modes are IR active and Raman active? How many peaks expected for each spectrum and why?arrow_forwardConsider the character table given below for the point group D3h, which includes molecules like cyclopropane and BF3. Suppose has been already determined a reducible representation for the collective motions of one such molecule, Γ(3N). What combination of irreps should we remove from it to account for translations & rotations, thereby yielding the purely vibrational representation, Γvib?arrow_forward
- Which of the fol lowing molecules can have (a) a pure rotational spectrum and (b) a rotational Raman spectrum: (i) HCI, Iii) N2O, (i ii) O3, (iv) SF4, (v) XeF4?arrow_forwardHow can I explain "why you obtain more XRD peaks when the symmetry is converted to lower symmetry. (Ex. From cubic to orthorhombic)"arrow_forwardWhat states of (i) benzene, (ii) naphthalene may be reached by electric dipole transitions from their (totally symmetrical) ground states?arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning


