
Concept explainers
(a)
Interpretation:
The difference in the proton NMR spectra of the given compound is to be stated.
Concept introduction:
The NMR stands for nuclear magnetic resonance. NMR spectroscopy deals with the interaction between
Answer to Problem 13.44AP
The difference in the proton NMR spectra of the compound A and compound B is that compound A contains a doublet and septet and compound B contains only singlet.
Explanation of Solution
The given compounds are shown below.
The formula used to determine NMR splitting is shown below.
Where
• n is the number of hydrogen atom on the adjacent atom.
The structure of compound A is shown below.
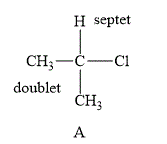
Figure 1
The compound A shown in Figure 1 contains six methyl protons and one methyne proton. The number of hydrogen atoms on adjacent atom of methyl protons
Therefore, the NMR spectra of methyl protons splits into a doublet as the number of peaks is
NMR spectra of methyne protons splits into septet as the number of peaks is
The structure of the compound
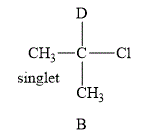
Figure 2
The compound B shown in Figure 2 contains six methyl protons. The number of hydrogen atoms on adjacent atom of methyl protons
The NMR spectra of methyl protons splits into singlet as the number of peaks is 0. As a result, there is no coupling. Therefore, the methyl protons give a singlet peak.
The compound A gives septet and doublet whereas compound B gives a singlet in proton NMR spectra.
(b)
Interpretation:
The difference in the proton NMR spectra of the given compound is to be stated.
Concept introduction:
The NMR stands for nuclear magnetic resonance. NMR spectroscopy deals with the interaction between electromagnetic radiation and the nucleus of an atom. NMR spectroscopy is used to determine the structural information about compounds.
Answer to Problem 13.44AP
The difference in the proton NMR spectra of the compound A and compound B is that compound A contains two triplets and compound B contains two triplets and one quintet (multiplet).
Explanation of Solution
The given compounds are shown below.
The formula used to determine NMR splitting is shown below.
Where
• n is the number of hydrogen atom on the adjacent atom.
The structure of compound A is shown below.
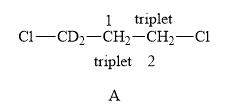
Figure 3
The compound A shown in Figure 3 contains four methylene protons. The methylene protons are adjacent to each other. Therefore, both of them couple with each other. The value of hydrogen atoms
Therefore, the NMR spectra of methyl protons of
Therefore, the NMR spectra of methyl protons of
The structure of compound B is shown below.
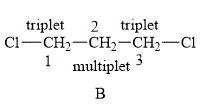
Figure 4
The compound B shown in Figure 4 contains six methylene protons. The methylene protons are adjacent to each other. The value of hydrogen atoms
Therefore, the NMR spectra of methyl protons of
Therefore, the NMR spectra of methyl protons of
The protons on the adjacent of
Therefore, the NMR spectra of methyl protons of
The compound A gives triplet at both the carbons whereas compound B gives triplet on
(c)
Interpretation:
The difference in the proton NMR spectra of the given compound is to be stated.
Concept introduction:
The NMR stands for nuclear magnetic resonance. NMR spectroscopy deals with the interaction between electromagnetic radiation and the nucleus of an atom. NMR spectroscopy is used to determine the structural information about compounds.
Answer to Problem 13.44AP
The difference in the proton NMR spectra of the compound A and compound B is that compound A contains a doublet and septet and compound B contains only singlet.
Explanation of Solution
The formula used to determine NMR splitting is shown below.
Where
• n is the number of hydrogen atom on the adjacent atom.
The structure of compound A and B are shown below.
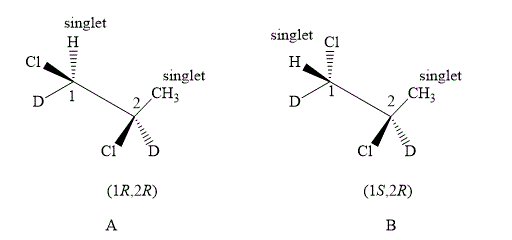
Figure 5
The compound A and B shown in Figure 5 both have similar spectrums. In the compound A, the value of number of methyl protons on adjacent to
Therefore, the NMR spectra of methyl protons of
Therefore, the NMR spectra of methyl protons of
Compound A and compound B both are diastereotopic. The chemical shift value of both the compounds is slightly different. The spectrum of both compounds is the same as there are diastereoisomers. Therefore, compound B also forms singlet on both the carbons.
The structure of compound C is shown below.
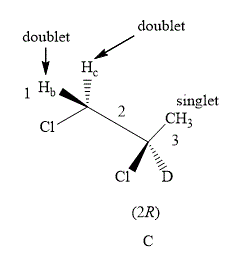
Figure 6
The compound C shown in Figure 6 contains five equivalent protons. The value of hydrogen atoms
Therefore, the NMR spectra of methyl protons of
Therefore, the NMR spectra of methyl protons of
The number of peaks of both
Therefore, the NMR spectra of methyl protons of
The compound A gives triplet at both the carbons whereas compound B gives triplet on
Want to see more full solutions like this?
Chapter 13 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





