
Concept explainers
(a)
Interpretation:
The product of the given reaction is to be predicted.
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydrogenation (Addition of
b)
Interpretation:
The products of the reaction to be predicted
Concept Introduction:
Reaction of
Nitration: The substitution of a nitro group for hydrogen on an aromatic ring.
(c)
Interpretation:
The product of the given reaction to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
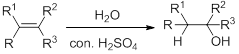
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (
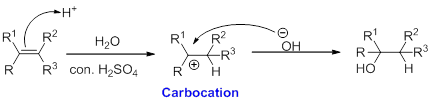
In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
Markovnikov’s rule: In the addition of
(d)
Interpretation:
The product of the given reaction to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydration:
When alkene is undergoes hydration with water in the presence of sulfuric acid which yields the alcohol. In this reaction, the water molecule will behave like a hydrogen halide to the alkene which gives the addition product this reaction is known as a hydration reaction.
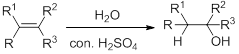
Alkene is reaction with water in the presence of sulfuric acid, first step is proton (

In hydration reaction, sulfuric acid is act as a proton donor, which is the driving force of the reaction. Hydration reaction will not go without acid (sulfuric acid).
Markovnikov’s rule: In the addition of
(e)
Interpretation:
The product of the given reaction to be predicted
Concept Introduction:
Addition reaction: A general reaction type in which a substance X-Y adds to the multiple bond of an unsaturated reactant to yield a saturated product that has only single bonds.
Hydrogenation (Addition of
Want to see the full answer?
Check out a sample textbook solution
Chapter 13 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
- Complete the following precipitation reactions using balanced chemical equations:arrow_forwardDraw the structure of the chemical species represented by numbers for the following reactions.arrow_forwardIdentify the oxidized reactant, the reduced reactant, the oxidizing agent, and the reducing agent in the following reactions:arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





