
Interpretation:
Charles’s Law needs to be explained.
Concept introduction:
Charles’s Law is also known as temperature-volume law.
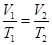
Where,
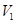 is the initial volume, and
is the initial volume, and
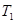 is the initial temperature, and
is the initial temperature, and
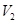 is the final volume, and
is the final volume, and
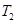 is the final temperature
is the final temperature
Explanation of Solution
According to the Charles’s Law, for a mixed mass of gas, the volume of gas decreases when temperature decreases and it increases when temperature is increased.
Charles’s Law is given by French physicist Jacques Charles. He studied how the volume of gas affects temperature on at constant pressure.
Charles’s Law declares that the volume of gas and temperature of the gas are directly proportional to each other at constant pressure.
Chapter 1 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
Chemistry: The Central Science (13th Edition)
Essential Organic Chemistry (3rd Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
General Chemistry: Principles and Modern Applications (11th Edition)
Chemistry: Structure and Properties
Introductory Chemistry (6th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





