
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 13, Problem 42P
Interpretation Introduction
Interpretation:
The structure for the adduct formed in the reaction between
Concept introduction:
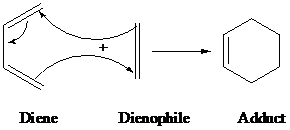
Diels-alder is a type of organic reaction in which substituted
The driving force of the reaction is the formation of new σ-bonds, which are energetically more stable than the π-bonds.
The general reaction of Diels-alder is as follows:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Give the structure, exclusive of stereochemistry, of the principal organic product formed on reaction of 2,3-dimethyl-1,3-butadiene with each of the following:(a) 2 mol H2, platinum catalyst(b) 1 mol HCl (product of 1,2-addition)(c) 1 mol HCl (product of 1,4-addition)(d) 1 mol Br2 (product of 1,2-addition)(e) 1 mol Br2 (product of 1,4-addition)(f) 2 mol Br2
The natural product halomon could theoretically arise from another naturally occurring compound known as myrcene. To accomplish
this, a biochemical process that could deliver the synthetic equivalent of BrCi to all three double bonds would be required. (Chem
Comm. 2014, 50, 13725)
(a) Using three molar equivalents of BrCL please provide a mechanism to account for the formation of the bracketed structure (you do
not need to show stereochemistry in this mechanism)
HB
(3 equiv)
myrcene
balomon
8.61a
Add curved arrow(s) to show the mechanism steps.
Edit Drawing
sits
Account for the following :(a) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.(b) Alkyl halides, though polar, are immiscible with water.(c) Grignard’s reagents should be prepared under anhydrous conditions.
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Prob. 44PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGPCh. 13 - Prob. 1QCh. 13 - Prob. 2QCh. 13 - Prob. 3QCh. 13 - Prob. 4QCh. 13 - Prob. 5Q
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw a structural formula for the alcohol formed by treating each alkene with borane in tetrahydrofuran (THF) followed by hydrogen peroxide in aqueous sodium hydroxide, and specify stereochemistry where appropriate. (a) (d) (b) (e) (c)arrow_forwardWrite the structure of the major organic product formed in the reaction of 1-pentene with each of the following: (a) Hydrogen chloride (b) Dilute sulfuric acid (c) Diborane in diglyme, followed by basic hydrogen peroxide (d) Bromine in carbon tetrachloride (e) Bromine in water (f) Peroxyacetic acid (g) Ozone (h) Product of part (g) treated with zinc and water (i) Product of part (g) treated with dimethyl sulfide (CH3)2Sarrow_forwardUsing cyclohexane as your starting material, show how you would synthesize each of the following compounds. (Onceyou have shown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) bromocyclohexane (b) cyclohexenearrow_forward
- Write structural formulas for the cyclohexadienyl cations formed from aniline (C6H5NH2) during(a) Ortho bromination (four resonance structures)(b) Meta bromination (three resonance structures)(c) Para bromination (four resonance structures)arrow_forward(a) Cyclohexa-1,3-diene can be converted into a tetrasubstituted haloalkane when reacted with bromine in ether. Write a balanced chemical equation for the reaction that occurs and state the expected observation. (b) Compound A and B are alkenes with the same molecular formula C5H10. Compound A is a branched-chain alkene while compound B is a straight-chain alkene. The reaction between compound A with hydrogen bromide produces major product C which is optically active. (i) Draw TWO (2) possible structures for compound B. (ii) Outline the mechanism for the reaction between compound A with hydrogen bromide to form major product C. (iii) Name the product formed when compound A undergoes bromination reaction.arrow_forward(a) When bromine is added to two beakers, one containing phenyl iso propyl ether and the other containing cyclohexene, the bromine color in both beakers disappears. What observation could you make while performing this test that would allow you to distinguish the alkene from the aryl ether? (b) Write any three methods in detail for the preparation of aldehyde from primary alcohol. (c) State the clemmenson reduction.arrow_forward
- What will be the color of the flame and the amount of soot if the following are ignited:(a) Hexane (b)Heptane(c) Cyclohexane (d) Cyclohexene (e) Benzene (f) Toluenearrow_forward11:43 Q1. (a) (c) (d) (b) Two stereoisomers of but-2-ene are formed when 2-bromobutane reacts with ethanolic potassium hydroxide. (i) Explain what is meant by the term stereoisomers. Library Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH3)2C=CH₂ Name of mechanism Mechanism (ii) Draw the structures and give the names of the two stereoisomers of but-2-ene. Stereoisomer 1 Name (iii) Name this type of stereoisomerism. Select Name Stereoisomer 2 When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. CH3 H₂C-C-CH3 + KOH Br Page 2 of 14 CH3 H3C-C-CH3 + KBr ОН State the role of the hydroxide ions in this reaction. Write an equation for the reaction that occurs when CH3CH₂CH₂CH₂Br reacts with an excess of ammonia. Name the organic product of this reaction. Equation Name of product 9,284 Photos, 1,166 Videos For You…arrow_forwardWhat are the expected kinetic and thermodynamic products from addition of one mole of Br, to the following dienes? (b)arrow_forward
- 8. (a) Benzene derivatives exhibit medium to strong absorption in UV-region. Explain why aniline and phenoxide ion have strong UV-absorptions.arrow_forward(a) Draw a Kekulé structure that shows how the reactive positions of anthracene are the ends of a diene, appropriate for a Diels–Alder reaction.(b) The Diels–Alder reaction of anthracene with maleic anhydride is a common organic lab experiment. Predict the product of this Diels–Alder reaction.arrow_forwardUsing cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctene (b) cyclooctane (c) trans-1,2-dibromocyclooctanearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY