
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13, Problem 45P
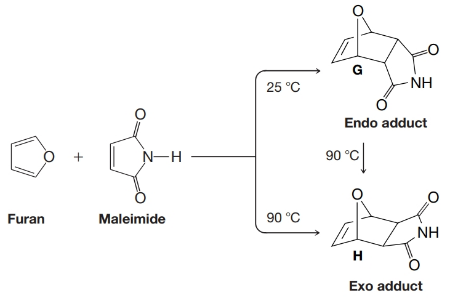
When furan and maleimide undergo a Diels–Alder reaction at 25°C, the major product is the endo adduct G. When the reaction is carried out at 90°C, however, the major product is the exo isomer H. The endo adduct isomerizes to the exo adduct when it is heated to 90°C. Propose an explanation that will account for these results.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Step 6: Now that you have determined the substrates and mechanism of a Diels-Alder reaction, you will learn how to recognize
when you should use the Diels-Alder reaction. In a synthesis reaction, if you are given a cyclohexene product with no other
obvious functional group transformations and an electron-withdrawing group two carbons away from the alkene, it is likely
made via the Diels-Alder reaction.
Deduce the structures of the starting materials to form the Diels-Alder adduct shown.
.....
CN
CN
Diene + Dienophile
Compounds P and Q can undergo a Diels-Alder reaction to form two
regioisomeric products R and S as shown in Figure 5.
OMe
O
C8H12O2
R
C8H12O2
S
Figure 5
Draw the chemical structures of regioisomeric compounds R and S.
Using possible resonance contributors of P and Q predict which of the two
regioisomers will be favoured in the reaction.
Using curly arrows, draw the mechanism for the reaction of P and Q to form the
dominant regioisomer you have predicted in your answer to part (ii) above.
The following is an example of a hetero Diels-Alder reaction, because a noncarbon atom (in this case, an N atom) is
involved in bond formation and bond breaking. Draw the curved arrows necessary to account for this transformation.
OCH3
OCH3
+
N-
Hydroquinone,
benzene,
25 °C, 90 min
86%
Chapter 13 Solutions
Organic Chemistry
Ch. 13 - Prob. 1PPCh. 13 - Prob. 2PPCh. 13 - Prob. 3PPCh. 13 - Practice Problem 13.4 From each set of resonance...Ch. 13 - Practice Problem 13.5 The following enol (an...Ch. 13 - Prob. 6PPCh. 13 - Practice Problem 13.7
Two compounds, A and B, have...Ch. 13 - Prob. 8PPCh. 13 - Prob. 9PPCh. 13 - Prob. 10PP
Ch. 13 - Prob. 11PPCh. 13 - Prob. 12PPCh. 13 - Prob. 13PPCh. 13 - Prob. 14PPCh. 13 - Prob. 15PPCh. 13 - Practice Problem 13.16
Diels–Alder reactions also...Ch. 13 - Prob. 17PPCh. 13 - Prob. 18PCh. 13 - What product would you expect from the following...Ch. 13 - Prob. 20PCh. 13 - Prob. 21PCh. 13 - Provide the reagents necessary for each of the...Ch. 13 - Prob. 23PCh. 13 - Prob. 24PCh. 13 - Prob. 25PCh. 13 - When 1-pentene reacts with N-bromosuccinimide...Ch. 13 - Prob. 27PCh. 13 - Prob. 28PCh. 13 - Prob. 29PCh. 13 - Prob. 30PCh. 13 - 13.31 Provide a mechanism that explains formation...Ch. 13 - 13.32 Provide a mechanism that explains formation...Ch. 13 - Treating either 1-chloro-3-methyl-2-butene or...Ch. 13 - Prob. 34PCh. 13 - Prob. 35PCh. 13 - Although both 1-bromobutane and 4-bromo-1-butene...Ch. 13 - Prob. 37PCh. 13 - Prob. 38PCh. 13 - Prob. 39PCh. 13 - Prob. 40PCh. 13 - Prob. 41PCh. 13 - Prob. 42PCh. 13 - Prob. 43PCh. 13 - Prob. 44PCh. 13 - 13.44 When furan and maleimide undergo a...Ch. 13 - Two controversial hard insecticides are aldrin and...Ch. 13 - Prob. 47PCh. 13 - Prob. 48PCh. 13 - Prob. 49PCh. 13 - Prob. 50PCh. 13 - Explain the product distribution below based on...Ch. 13 - Mixing furan (Problem 13.44) with maleic anhydride...Ch. 13 - Prob. 53PCh. 13 - Prob. 54PCh. 13 - Prob. 1LGPCh. 13 - Prob. 2LGPCh. 13 - Prob. 1QCh. 13 - Prob. 2QCh. 13 - Prob. 3QCh. 13 - Prob. 4QCh. 13 - Prob. 5Q
Additional Science Textbook Solutions
Find more solutions based on key concepts
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
8.63 Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g ...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Q4. Which property of rubbing alcohol is a chemical property?
a) Density (0.786 g/cm3)
b) Flammability
c) Bo...
Chemistry: A Molecular Approach (4th Edition)
Ethanol can be produced commercially by the hydration of ethylene: C2H4 + H2O -* C2H5OH Some of the product is ...
Elementary Principles of Chemical Processes, Binder Ready Version
Rank the given solvents in decreasing order of their ability to dissolve each compound. Solutes
Organic Chemistry (9th Edition)
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following is an example of a type of reaction known as a Diels-Alder reaction 1,3-Pentadiene Ethylene 3-Methylcyclohexene (a racemic mixture) The Diels-Alder reaction between a diene and an alkene is quite remarkable in that it is one of the few ways that chemists have to form two new carbon-carbon bonds in a single reaction. Given what you know about the relative strengths of carbon-carbon sigma and pi bonds, would you predict the Diels-Alder reaction to be exothermic or endothermic? Explain your reasoning.arrow_forwardPredict the major product of the following Diels-Alder reactions with the correct regiochemistry and stereochemistry. If an enantiomer is also made, indicate the enantiomer by adding “+ EN” beside the major product. Please explain step by step.arrow_forwardExp3: Diels-Alder reaction Why does cyclopentadiene regularly form a dimer? How to break the dimer into monomers? Does the diene in this reaction act as the nucleophile or the electrophile? Is the maleic anhydride a nucleophile or an electrophile in this reaction? Explain your reasoning.arrow_forward
- 1,3-Butadiene is a gas at room temperature that requires a gas-handling apparatus to use in a Diels-Alder reaction. Butadiene sulfone is a convenient substitute for gaseous 1,3-butadiene. This sulfone is a solid at room temperature (mp 66°C), and when heated above its boiling point of 110°C, it decomposes by a reverse Diels-Alder reaction to give cis-1,3-butadiene and sulfur dioxide. Draw Lewis structures for butadiene sulfone and SO, then show by curved arrows the path of this reaction, which resembles a reverse Diels-Alder reaction. 140°C SO2 Butadiene sulfone 1,3-Butadiene Sulfur dioxidearrow_forwardwhen furan and maleimide undergo a diels alder reaction at 25 celcius the major product is the endo adduct when the reaction is carried out at 90 celcius the major product is exo isomer the endo adduct isomerizes to the exo adduct when it is heated to 90 celcius. propose explanation that will account for these results and what is the mechanism of this reactionarrow_forwardA hetero Diels-Alder reaction is a variation of the Diels-Alder reaction in which one or more carbon atoms of the diene and/or the dienophile are replaced by other atoms such as oxygen or nitrogen. With this in mind, propose a mechanism consistent with the following transformation in which a multifunctional acyclic reactant produces a macrocyclic (large ring) product. 195°C. 2 Ph Ph- Ph IIarrow_forward
- The following triene undergoes an intramolecular Diels-Alder reaction to give a bicyclic product. Propose a structural formula for the product. Account for the observation that the Diels-Alder reaction given in this problem takes place under milder conditions (at lower temperature) than the analogous Diels-Alder reaction shown in Problem 20.34.arrow_forwardPlease answer both parts to the questions completely. I will rate the answer afterwards. For a diels-alder reaction between anthracene and maleic anhydride, are the exo and endo forms of product 9,10-dihydroanthracene-9,10-ɑ,β-succinic acid anhydride different stereoisomers or are they the same molecule? Explain your answer by drawing the molecule. Would it be favorable to get a 1,4-adduct of anthracene and maleic anhydride? Why or why not? If the 1,4-adduct of anthracene and maleic anhydride had formed, would it have different exo and endo isomers? Yes or no and why?arrow_forward74. When piperidine undergoes the series of reactions shown here, 1,4-pentadiene is obtained as the product. When the four different methyl-substituted piperidines undergo the same series of reactions, each forms a different diene: 1,5-hexadiene; 1,4-pentadiene; 2-methyl-1,4-pentadiene; and 3-methyl-1,4-pentadiene. Which methyl-substituted piperidine forms which diene? 1. еxcess CHз!/К2CO3 2. Ag20, H20 3. Д 1. еxcess CHз!/K2CO3 2. Ag20, H20 CH3NCH,CH,CH;CH=CH2 3. A CH2=CHCH2CH=CH2 || `N' H CH3 1,4-pentadiene piperidinearrow_forward
- A2 What is the inverse electron demand Diels–Alder reaction? Please pick a pair of a diene and a dienophile from the following dienes and dienophile that will undergo this type of reaction. Please show how this reaction works using Frontier Molecular Orbitals. What is the reaction product?arrow_forwardFuran and maleimide undergo a Diels–Alder reaction at 25 °C to give the endo isomer of the product. When the reactiontakes place at 90 °C, however, the major product is the exo isomer. Further study shows that the endo isomer of theproduct isomerizes to the exo isomer at 90 °C.furan: O maleimide:OON H(a) Draw and label the endo and exo isomers of the Diels–Alder adduct of furan and maleimide.(b) Which isomer of the product would you usually expect from this reaction? Explain why this isomer is usually favored.(c) Examine your answer to (b) and determine whether this answer applies to a reaction that is kinetically controlled orone that is thermodynamically controlled, or both.(d) Explain why the endo isomer predominates when the reaction takes place at 25 °C and why the exo isomer predominates at 90 °Carrow_forwardThe following triene undergoes an intramolecular Diels-Alder reaction to give a bicyclic product. Propose a structural formula for the product. Account for the observation that the Diels-Alder reaction given in this problem takes place under milder conditions (at lower temperature) than the analogous Diels-Alder reaction 0"C Diels-Alder adductarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY