
Concept explainers
(a)
Interpretation:
The number of nodal planes perpendicular to the bonding axes of each of the three
Concept introduction:
Overlap of atomic orbitals (AOs) can be thought of as wave interference. It can be constructive or destructive. Constructive interference increases the electron density between the two nuclei (an antinode) and results in a molecular orbital (MO) of lower energy. The phases of the overlapping orbitals are the same in this case. Destructive interference decreases the electron density between the two nuclei and results in an MO of higher energy. The phases of such AOs are opposite. Since the electron density between the two nuclei decreases, there is a node (or a nodal plane) between the two atoms.
Answer to Problem 14.27P
The number of nodal planes is one for MO A, six for MO B, and none for MO C.
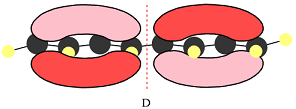
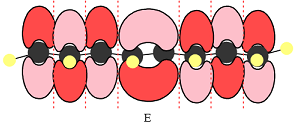
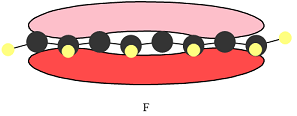
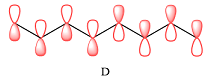
The order of increasing energy of the three MOs is
Explanation of Solution
The phases of the wave function of the two AOs on either side of a nodal plane are opposite each other. This results in destructive interference reducing the electron density to zero at the nodal plane.
Therefore, the nodal planes in the three MOs are:



The energy of the MO increases with the number of nodes. Therefore, the order of increasing energy of the three orbitals is
When atomic orbitals with different phases overlap, a node (zero electron density) is formed at the center, increasing the energy of the MO.
(b)
Interpretation:
The p orbital AO contributions on each carbon atom that would give rise to each MO are to be drawn.
Concept introduction:
The p orbitals that contribute to
Answer to Problem 14.27P
The p orbitals that contribute to each of the three MOs are
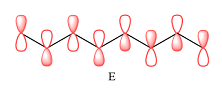
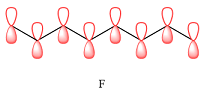
Explanation of Solution
In case of D, there are two four-center MOs. Therefore, p orbitals that contribute to it must have the same phase. The second MO has the opposite phase. The p AOs that contribute to this MO will all have the same phase, but it will be opposite to that of the first group.
Therefore, the p orbitals that contribute to MOs shown in A are

E shows a total of five MOs. The first three from left are present in individual atoms. The fourth one is a two-center MO, followed again by three individual MOs. Only the AOs of C4 and C5 must have matching phases. All other adjacent AOs will have mismatched phases.
Therefore, the p orbitals contributing to the MOs as shown in D are

In case of F, a single MO covers all eight carbon atoms. Therefore, the contributing AOs must all have the same phase.

The p orbitals contributing to each MO are determined on the basis of the phases and the presence of an adjacent nodal plane.
(c)
Interpretation:
Each internuclear region is to be identified as having a bonding or an antibonding type of interaction.
Concept introduction:
A bonding interaction arises when the phases of the interacting AOs are the same. This increases the electron density between the two nuclei and lowers the energy of the MO. An antibonding interaction arises when the phases of the interacting AOs are different. This decreases the electron density between the two nuclei to zero at the center and increases the energy of the MO.
Answer to Problem 14.27P
The bonding (
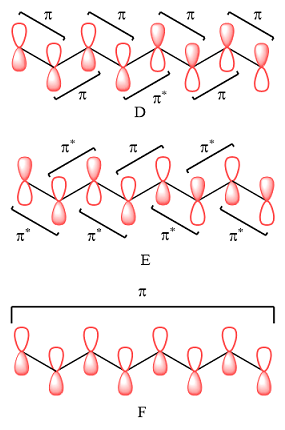
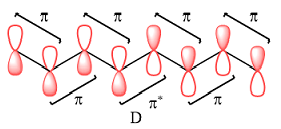
Explanation of Solution
A bonding interaction requires p orbitals of the same phase on adjacent atoms. An antibonding interaction requires the interacting p orbitals to be of opposite phases. An antibonding interaction leads to a nodal plane between the two atoms.
Therefore, in D, there are six bonding interactions. There is only one antibonding interaction between C4 and C5 orbitals.
In case of E, there is only one bonding interaction between C4 and C5 orbitals. All other interactions are antibonding interactions.
In case of F, all are bonding interactions.
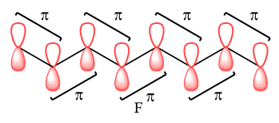
The type of interaction is determined on the basis of the phases of interacting AOs.
(d)
Interpretation:
Whether each MO is overall bonding, nonbonding, or antibonding is to be determined on the basis of the answer to part (c).
Concept introduction:
If the number of bonding interactions are more than the number of antibonding interactions, the MO is overall bonding. If the number of antibonding interactions is more than that of bonding interactions, the MO is overall antibonding. If the numbers are equal or there are no interactions, the MO is overall nonbonding.
Answer to Problem 14.27P
The MO shown in D is overall bonding. MO E is overall antibonding. MO F is overall antibonding. MO C is overall bonding.
Explanation of Solution
There are six bonding and only one antibonding interaction in this case. Therefore, the MO shown in D is overall bonding.
In the case of E, there is only one bonding interaction and six antibonding interactions. Therefore, MO E is overall antibonding.
All interactions in MO C are bonding interactions. Therefore, this MO is overall bonding.
The overall character of a multicenter MO is determined by the numbers of bonding and antibonding interactions.
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- How would you draw a molecular orbital diagram of butadiene showing the energies and occupancy ofthe orbitals in the ground state? How would you estimate the total pi binding energy of the molecule?arrow_forwardSolve for the delocalization and overall electronic energy for C4H4 (considering a single p orbital on each carbon) ? How does this compare to benzene?arrow_forwardThe FEMO theory of conjugated molecules is crude and marginally better results are obtained with simple Huckel theory. (a) For a linear conjugated polyene with each of N carbon atoms contributing an electron in a 2porbital, the energies Ek of the resulting π molecular orbitals are given by: Ek = α + 2β cos kπ/N+1 k = 1,2,3, ... N Use this expression to estimate the resonance integral β for the series consisting of ethene, butadiene, hexatriene, and octatetraene given that ult raviolet absorptions from the HOMO, which is a bonding π orbital, to the LUMO, which is an antibonding π* orbital, occur at 61 500, 46 080, 39 750,and 32 900 cm- 1, respectively. (b) Calculate the π-electron delocalization energy, Edelcc = Eπ -n(α + β). of octatetraene, where Eπ, is the total π-electron binding energy and n is the total number of π-electrons.arrow_forward
- he structure of 1,2-propadiene (allene) is shown to the right. Q.) Predict all approximate bond angles in this molecule.arrow_forwardPropose structures for molecules that meet the following descriptions: Contains two sp2 hybridized carbons and two sp3 hybridized carbons.arrow_forwardThree of the four molecular orbitals for cyclobutadiene are pictured here. Place them in order of increasing energy. (See Figures 9.13, 9.15, 9.16, and 9.18 and the relation of orbital energy and nodes.)arrow_forward
- For each of the following molecules, construct the MOs from the 2pz atomic orbitals perpendicular to the plane of the carbon atoms. (a) Cyclobutadiene HC (b) Allyl radical Indicate which, if any, of these orbitals have identical energies from symmetry considerations. Show the number of electrons occupying each MO in the ground state, and indicate whether either or both of the molecules are paramagnetic. Assume that the C atoms in the allyl radical are all sp2 hybridized.arrow_forwardHow many anti-bonding pi-type molecular orbitals does 1,3-butadiene contain?arrow_forwardWhich of the following represents the lowest energy pi molecular orbital for cyclopentadiene?arrow_forward
- Let us construct the molecular orbital diagram of ethylene (in pieces). a. First, construct the MO diagram of linear carbene (CH2). Draw pictures of all 6 orbitals b. Now bend the carbene to a bond angle of about 120°. How does this change your MO diagram? Draw pictures of all 6 orbitals. c. Now bring two of these carbene molecules together to make ethylene. Draw pictures of all 12 orbitals.arrow_forwardWhich bond-line structure is represented by the Newman projection below?arrow_forwardWrite the ground state (the lowest energy) electronic configuration of each molecule or molecular ion below: a. H2- b. N2 c. O2 Please label molecular orbitals with appropriate “g” (“gerade”) and “u” (“ungerade”) subscripts.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




