
Concept explainers
To enhance the effective surface, and hence the chemical reaction rate, catalytic surfaces often take the form of porous solids. One such solid may be visualized as consisting of a large number of cylindrical pores, each of diameter D and length L. 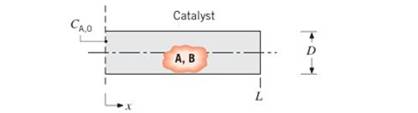
Consider conditions involving a gaseous mixture of A and B for which species A is chemically consumed at the catalytic surface. The reaction is known to be first order, and the rate at which it occurs per unit area of the surface may be expressed as
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Fundamentals of Heat and Mass Transfer
- Your team has invented a compound for use in refrigeration processes, R−X. You have asked your technician to characterize the pure component vapor-liquid equilibrium of the fluid at 300 K, and he has faxed you the results below. Unfortunately, the quality of the fax is low so that you are unable to distinguish if the properties correspond to those of the liquid or vapor phase. Using only the provided data, determine which properties correspond to the liquid and vapor phase. Also be sure to explain how you came up with your answer. T = 300 K P = 7.0282 bar V = 0.83357cm3/g V = 29.246cm3/g U = 392.71 kJ/kg U = 236.60 kJ/kgarrow_forwardA substance has a melting point of 20°C and a heat of fusion of 2.4 × 10 to power of ((4)) J/kg. The boiling point is 150°C and the heat of vaporization is 4.8 × 10 to power of ((4)) J/kg at a pressure of 1.0 atm. The specific heats for the solid, liquid, and gaseous phases are 600 J/(kg • K), 1000 J/(kg • K), and 400 J/(kg • K), respectively. The quantity of heat required to raise the temperature of 3.00 kg of the substance from 9°C to 98°C, at a pressure of 1.0 atm, is closest toarrow_forwardShown to the right is the solid-liquid phase behavior for mixtures of component A and component B at 1 atm. Use the phase diagram to answer the following questions. a) Specify the melting point of substance B at 1 atm b) What is the maximum composition (mole fraction) of component B that is possible for the mixture to exist in phase S2? At what temperature does this occur? c) 20mol of component A and 180mol of component B are mixed at 350°C and 1 atm. The mixture is cooled at constant pressure to 200°C. i) What phases are present at the final state? ii) What is the composition of each phase at the final state? iii) What is the number of moles of each phase at the final state?arrow_forward
- a product having a moisture content of 60˚C (wet basis) is dried by tunnel type drying at a rate of 10 kg / hr. Drying air is supplied at a rate of 1000 kg air / hour at 50˚C and 10% RH, and drying out at 25˚C, with equilibrium conditions with the product at 40% RH. determine the moisture content of the product that comes out of the dryer, as well as the activity of the product. a. product moisture content = % (wet basis) b. water activity =arrow_forwardCompute diffusion coefficients for the interdiffusion of carbon in both (a) α-iron (BCC) and (b) γ-iron (FCC) at 1000˚C. Assume that D0 for the interdiffusion of carbon in α-iron and in γ-iron are 1.1 × 10-6 and 2.3 × 10-5 m2/s, respectively, and that Qd are 80 and 148 kJ/mol, respectively.arrow_forward**JUST PART C PLEASE** The pressure in a pipeline that transports helium gas at a rate of 5 lbm/s is maintained at 14.7 psia by venting helium to the atmosphere through a 0.25-inch internal diameter vertical tube that extends10 ft into the air. Helium and atmospheric air are at 60F. The diffusion coefficient for a mixture of helium with air is 2.79 ft2/h at 77oF. Assume the mole fraction of helium in the atmosphere is negligible, and the mole fraction of air in the helium pipeline is negligible. Determine the following:(a) The mass flow rate of helium flowing to the atmosphere through the tube.(b) The mass flow rate of air that infiltrates into the pipeline.(c) The net mass flow rate in the vertical tube.arrow_forward
- The outer surface of a steel gear is to be hardened by increasing its carbon content. The carbon is to be supplied from an external carbon-rich atmosphere, which is maintained at an elevated temperature. A diffusion heat treatment at 850 °C (1123 K) for 30 min increases the carbon concentration to 0.90 wt% at a position 1.0 mm below the surface. Estimate the diffusion time required at 700 °C (973 K) to achieve this same concentration also at a 1.0-mm position. Assume that the surface carbon content is the same for both heat treatments, which is maintained constant. Use the diffusion data in Table 5.2 for C diffusion in -Fe. Hint: before trying to solve this using brute force, consider what is changing from the first process to the second process and how that impacts the equation for diffusion.arrow_forwardA thin plastic membrane is used to separate helium from a current.gaseous. Under steady-state conditions, a concentration of helium in the membrane is known to be 0.02 and 0.005 kmol/m³ on the inner and outer surfaces, respectively. If the membrane has a thickness of 1 mm and the coefficient ofbinary diffusion of helium to plastic is 10^(-9) m²/ s, what is the value of the flow diffusive?arrow_forwardGaseous hydrogen weakens the mechanical strength of cast iron. this phenomenon often occurs in cast iron pressure vessels containing 100% gas hydrogen. H2 gas dissolves in metallic iron and diffuses into solid non-porous iron by an interstitial diffusion mechanism. H2 gas does not need to penetrate far into the iron to have a negative effect on the mechanical strength of iron. In the present situation, 100% of H2 gas at 1.0 atm and 100°C is contained within a 1.0 m internal diameter and wall thickness of 2.0 cm. The solubility of hydrogen in iron in 100°C is 2.2x10-7 mol of H/g Fe atoms. The diffusion coefficient of atoms of hydrogen in solid iron is 124.0x10-9 cm2 /sec at 100°C. Initially, there are no H atoms in solid iron. How many hours will it take for the hydrogen level inside the iron metal reaches 1.76x10-7 mol H atoms/g Fe at a depth of 0.1 cm from the surface exposed to hydrogen gas?arrow_forward
- A mechanism for hardening steel is called carburization. To achieve this process, the piece of steel is exposed to an atmosphere rich in hydrocarbon such as methane (CH4). Consider a steel with a carbide concentration of 0.25wt%, which must be treated at 950˚C. If the carbon concentration at the surface is suddenly increased to 1.20wt%, how long does it take for a penetration of 0.5mm from the surface to reach a concentration of 0.80wt%? . The diffusion coefficient for carbon in iron at this temperature is 1.6 x 10-11 m2s-1. Assume that the piece of steel is semi-finite. Use the table below.arrow_forwardA large piece of biological tissue has to be kept at a temperature as low as possible without freezing (ice formation). The water in the tissue has several solutes dissolved in it and the effective molecular weight of the dissolved solutes is 50. The weight of all dissolved solutes combined is 10% of the weight of water. The latent heat of fusion of water is 335 kJ/kg and the molecular weight of water is 18. What is the lowest temperature that this tissue can be stored at, without ice formation?arrow_forwardA plate of iron is exposed to a carburizing atmosphere on one side and a decarburizing atmosphere on the other side at 850 oC. If a condition of steady state is achieved, calculate the diffusion flux of carbon through the plate if the concentrations of carbon at positions of 5 and 10 mm beneath the carburizing surface are 1.4 and 0.8 kg/m3, respectively. Assume a diffusion coefficient of 3 x 10 -11 m2/s at this temperature.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





