
Concept explainers
(a)
Interpretation:
The substitutive name and common name of
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing IUPAC name from the structural formula are given below.
- First, identify the longest carbon chain.
- The next step is to identify the groups attached to the longest chain.
- Identify the position, location, and a number of the substituents bonded to the carbon chain.
- Use prefix di, tri, tetra if the same type of substituents is present.
- Name the substituents in alphabetical order.
Answer to Problem 14.3P
The common name of the compound
Explanation of Solution
The compound
The compound
The number on the carbon atoms in the compound is shown below.
The compound is
The common name of the compound
(b)
Interpretation:
The substitutive name and common name of
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
- First, identify the longest carbon chain.
- The next step is to identify the groups attached to the longest chain.
- Identify the position, location, and a number of the substituents bonded to the carbon chain.
- Use prefix di, tri, tetra if the same type of substituents is present.
- Name the substituents in alphabetical order.
Answer to Problem 14.3P
The common name of the compound
Explanation of Solution
The compound
The compound
The number on the carbon atoms in the compound is shown below.
The compound is alkyne. Therefore, -yne will be present as the suffix. The triple bond is present on the fifth carbon atom. The compound has ten-carbon atom long chain. Therefore, the substitutive name of the compound is
The common name of the compound
(c)
Interpretation:
The substitutive name of compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
- First, identify the longest carbon chain.
- The next step is to identify the groups attached to the longest chain.
- Identify the position, location, and a number of the substituents bonded to the carbon chain.
- Use prefix di, tri, tetra if the same type of substituents is present.
- Name the substituents in alphabetical order.
Answer to Problem 14.3P
The substitutive name of the given compound is
Explanation of Solution
The structure of the given compound is shown below.
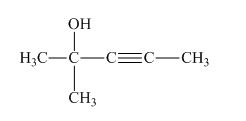
Figure 1
The compound has five carbon atom in the longest chain. Therefore, pent- will be used in the root name. The number of the carbon atoms will be started from the carbon atom which gives the lowest number to hydroxyl group in the chain.
The number on the carbon atoms in the compound is shown below.
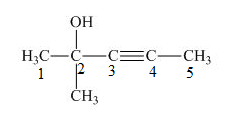
Figure 2
The compound has methyl group and hydroxyl group. Therefore, -yne will be present as primary suffix and –ol will be present on as secondary suffix. The methyl group is resent at the second carbon. Therefore, methyl will be used as prefix. The triple bond is present on the third carbon atom and hydroxyl group is present on the second carbon atom. Therefore, the substitutive name of the compound is
The substitutive name of the given compound is
(d)
Interpretation:
The substitutive name of compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
- First, identify the longest carbon chain.
- The next step is to identify the groups attached to the longest chain.
- Identify the position, location, and a number of the substituents bonded to the carbon chain.
- Use prefix di, tri, tetra if the same type of substituents is present.
- Name the substituents in alphabetical order.
Answer to Problem 14.3P
The substitutive name of the compound is
Explanation of Solution
The structure of the given compound is shown below.
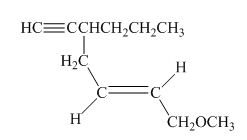
Figure 3
The compound has seven carbon atom in the longest chain. Therefore, hept- will be used in root name. The number of the carbon atoms will be started from the carbon atom which gives lowest number to propyl group in the chain.
The number on the carbon atoms in the compound is shown below.
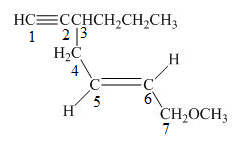
Figure 4
The compound has propyl group and methoxy group. Therefore, methoxy and propyl will be present as suffix. The propyl group is present at the third carbon. The triple bond is present on the first carbon atom and double bond is present at the fifth carbon atom. Therefore, the substitutive name of the compound is
The substitutive name of the given compound is
(e)
Interpretation:
The substitutive name of compound is to be stated.
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
- First, identify the longest carbon chain.
- The next step is to identify the groups attached to the longest chain.
- Identify the position, location, and a number of the substituents bonded to the carbon chain.
- Use prefix di, tri, tetra if the same type of substituents is present.
- Name the substituents in alphabetical order.
Answer to Problem 14.3P
The substitutive name of the given compound is
Explanation of Solution
The structure of the given compound is shown below.
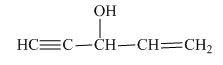
Figure 5
The compound has five carbon atom in the longest chain. Therefore, pent- will be used in root name. The number of the carbon atoms will be started from the carbon atom which gives lowest number to double bond in the chain.
The number on the carbon atoms in the compound is shown below.
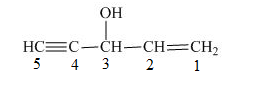
Figure 6
The compound has hydroxyl group. Therefore, hydroxyl will be present as prefix. The hydroxyl group is present at the third carbon. The triple bond is present on the fifth carbon atom and double bond is present at the first carbon atom. Therefore, the substitutive name of the compound is
The substitutive name of the given compound is
Want to see more full solutions like this?
Chapter 14 Solutions
Organic Chemistry
- Arrange these compounds in order of increasing boiling point (values in C are 42, 24, 78, and 118). (a) CH3CH2OH (b) CH3OCH3 (c) CH3CH2CH3 (d) CH3COOHarrow_forwardAn unknown hydrocarbon Q has a formula C6H12. Q Reacts with osmium tetroxide to give a diol R. When oxidized with KMnO4 in an acidic medium, Q gives two products. One product is propanoic acid and the other a ketone S. Provide reaction equations to identify the possible structures of Q, R and S.arrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forward
- Write the structure of the major products in the following reactions and name them.arrow_forwardDraw structures for cis-2,3-dibromo-2-pentene and 2,3-dibromo-2-pentene. Provide wedges and dashes and include all H atoms.arrow_forwardDraw all the alcohol having the formula C5H12Land which are choralarrow_forward
- When compound CH3-C(CH3)=CH-CH2-CH(CH3)2 is the SUBSTRATE (reactant) in a HALOGENATION reaction with Br2 (and CH2Cl2 as the solvent), a correct condensed structural formula for the MAJOR SUBSTRATE PRODUCT of this reaction is:arrow_forwardDraw the structural formulas of compounds A, C, D, E and F and draw the isomer of B, with the explaination on which one would be the major product and why.arrow_forwardDraw structural formulas for the cis and trans isomers of hydrindane. Show each ring in its most stable conformation. Which of these isomers is more stable?arrow_forward
- Determine the DOU for the following molecules and suggest a structure for each. C5H7Br2ONarrow_forwardbenzene, C6H6, gives thecompound special stability.(a) By using data in Appendix C, compare the heat of combustionof 1.0 mol C6H6(g) to the heat of combustion of 3.0 mol acetylene,C2H2(g). Which has the greater fuel value, 1.0 mol C6H6(g) or 3.0mol C2H2(g)? Are your calculations consistent with benzene beingespecially stable?arrow_forwardGive the skeletal structure of the reacting alkene and the reagents the must eb used to produce BrCH2CHOHCH2Clarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
