
(a)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(a)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
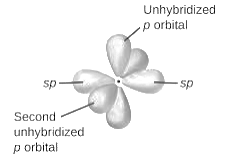
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 8
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 5 shows that the hybridization of central an atom is sp3d and geometry is Trigonal bipyramidal.
(b)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(b)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
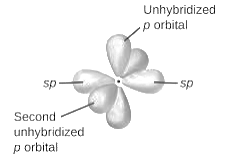
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 7
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 5 shows that the hybridization of central an atom is sp3d and geometry is Trigonal bipyramidal.
(c)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(c)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
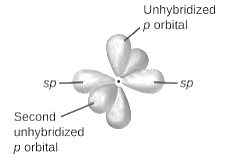
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom, present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 6
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 5 shows that the hybridization of central an atom is sp3d and geometry is Trigonal bipyramidal.
(d)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(d)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
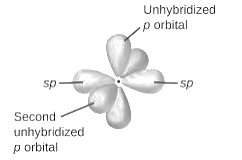
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom.
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 5
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 5 shows that the hybridization of central an atom is sp3d and geometry is Trigonal bipyramidal.
(a)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(a)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
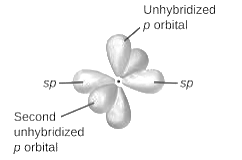
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 7
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 6 shows that the hybridization of central an atom is sp3d2 and geometry is octahedral.
(b)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(b)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
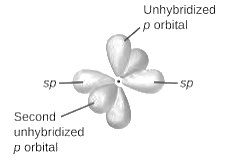
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons = 8
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 6 shows that the hybridization of central an atom is sp3d2 and geometry is octahedral.
(c)
Interpretation:
The expected hybridization of the central an atom in
Concept Introduction:
When two atomic orbitals combine with each other to produce hybrid orbitals, redistribution of energy of orbitals of distinct an atom s to form orbitals with equal energy occurs. This process is known as hybridization and the formed new orbitals are known as hybrid orbitals.
(c)
Answer to Problem 20E
The expected hybridization of the central an atom in
Explanation of Solution
On the basis of types of orbitals involved in mixing, different hybridization is classified as sp, sp2, sp3, sp3d, sp3d2, sp3d3.
sp-hybridization: It is formed when one s and one p orbital mix with each other in same shell of an an atom to produce two new equal orbitals. This hybridization takes place in linear molecules.
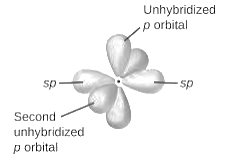
sp2-hybridization: It is formed when one s and two p orbital mix with each other in same shell of an an atom to produce three new equal orbitals. This hybridization takes place in molecules exhibits trigonal planar geometry.

sp3-hybridization: It is formed when one s and three p orbital mix with each other in same shell of an atom to produce four new equal orbitals. This hybridization takes place in molecules exhibits tetrahedral geometry. In this hybridization, no p-unhybridized orbital is present as all are hybridized and form four sigma bonds.
Now, for an atom , present in the structure compound, the sum of the number of bonded an atom s and number of lone pairs present on it results its steric number. The value of steric number tells about the hybridization of central an atom .
| Steric Number | Hybridization | Structure |
| 2 | sp | Linear |
| 3 | sp2 | Trigonal Planar |
| 4 | sp3 | Tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | Octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
In
Number of valence electrons =6
The expression for calculating number of electron pairs is:
Where, X = number of electron pairs
V = valence electrons of central an atom
M = number of monovalent an atom s
C = charge on compound.
In
Number of lone pairs = 0
Steric number 6 shows that the hybridization of central an atom is sp3d2 and geometry is octahedral.
Want to see more full solutions like this?
Chapter 14 Solutions
Chemical Principles
- Give the expected hybridization of the central atom for the molecules or ions in Exercises 81 and 87 from Chapter 3.arrow_forwardWhat hybrid orbitals would be expected for the central atom in each of the following molecules or ions?arrow_forwardDo lone pairs about a central atom affect the hybridization of the central atom? If so, how?arrow_forward
- Compare and contrast the molecular orbital and ionic bonding descriptions of LiF.arrow_forwardIdentify the hybrid orbitals used by antimony in SbCl5 and in SbCl6, the ion formed from the reaction of SbCl5 and Cl. Explain your choices.arrow_forward7.57 What observation about molecules compels us to consider the hybridization of atomic orbitals?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





