
(a)
Interpretation: The reason of stability of
Concept Introduction: Molecular orbital theory explained the bonding, magnetic and spectral properties of molecule. It is based on the formation of molecular orbitals by the combination of atomic orbitals. On the basis of energy and stability these molecular orbitals can be further classified in three types:
- Bonding molecular orbitals (BMO): They have lesser energy than atomic orbital therefore more stable compare to atomic orbital.
- Antibonding molecular orbitals (ABMO): They have higher energy than atomic orbital therefore less stable compare to atomic orbital.
- Non-bonding molecular orbitals (NBMO): They have same energy as atomic orbital.
Molecular orbital diagrams represents the distribution of electrons in different molecular orbitals in increasing order of their energy. Hence lower energy molecular orbitals occupy first then only electron moves in higher energy orbitals.
(a)
Answer to Problem 39E
Since the bond order of
Explanation of Solution
To draw the energy level and Molecular orbital diagram for
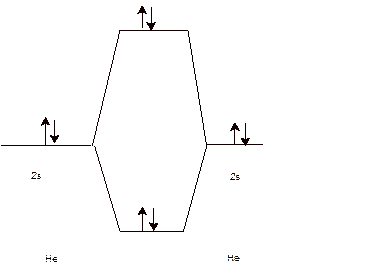
Calculate bond order:
Since the bond order of
(b)
Interpretation: The reason of paramagnetic nature of
Concept Introduction: Molecular orbital theory explained the bonding, magnetic and spectral properties of molecule. It is based on the formation of molecular orbitals by the combination of atomic orbitals. On the basis of energy and stability these molecular orbitals can be further classified in three types:
- Bonding molecular orbitals (BMO): They have lesser energy than atomic orbital therefore more stable compare to atomic orbital.
- Antibonding molecular orbitals (ABMO): They have higher energy than atomic orbital therefore less stable compare to atomic orbital.
- Non-bonding molecular orbitals (NBMO): They have same energy as atomic orbital.
Molecular orbital diagrams represents the distribution of electrons in different molecular orbitals in increasing order of their energy. Hence lower energy molecular orbitals occupy first then only electron moves in higher energy orbitals.
(b)
Answer to Problem 39E
Only
Explanation of Solution
The molecular orbital electronic configuration of
Only
(c)
Interpretation: The reason of large
Concept Introduction: Molecular orbital theory explained the bonding, magnetic and spectral properties of molecule. It is based on the formation of molecular orbitals by the combination of atomic orbitals. On the basis of energy and stability these molecular orbitals can be further classified in three types:
- Bonding molecular orbitals (BMO): They have lesser energy than atomic orbital therefore more stable compare to atomic orbital.
- Antibonding molecular orbitals (ABMO): They have higher energy than atomic orbital therefore less stable compare to atomic orbital.
- Non-bonding molecular orbitals (NBMO): They have same energy as atomic orbital.
Molecular orbital diagrams represents the distribution of electrons in different molecular orbitals in increasing order of their energy. Hence lower energy molecular orbitals occupy first then only electron moves in higher energy orbitals.
(c)
Answer to Problem 39E
Since the bond order for
Explanation of Solution
The molecular orbital electronic configuration of
Calculate bond order:
Since the bond order for
(d)
Interpretation: The reason of more stability of
Concept Introduction: Molecular orbital theory explained the bonding, magnetic and spectral properties of molecule. It is based on the formation of molecular orbitals by the combination of atomic orbitals. On the basis of energy and stability these molecular orbitals can be further classified in three types:
- Bonding molecular orbitals (BMO): They have lesser energy than atomic orbital therefore more stable compare to atomic orbital.
- Antibonding molecular orbitals (ABMO): They have higher energy than atomic orbital therefore less stable compare to atomic orbital.
- Non-bonding molecular orbitals (NBMO): They have same energy as atomic orbital.
Molecular orbital diagrams represents the distribution of electrons in different molecular orbitals in increasing order of their energy. Hence lower energy molecular orbitals occupy first then only electron moves in higher energy orbitals.
(d)
Answer to Problem 39E
Since there are more anti-bonding electrons in
Explanation of Solution
The molecular orbital electronic configuration of
Number of electrons in N = 7
Number of electrons in O = 8
Total number of electrons in
Total number of electrons in
Calculate bond order:
Since there are more anti-bonding electrons in
Want to see more full solutions like this?
Chapter 14 Solutions
Chemical Principles
- Describe the hybridization around the central atom and the bonding in SCl2 and OCS.arrow_forwardWhat modification to the molecular orbital model was made from the experimental evidence that B2 is paramagnetic?arrow_forwardThe sulfamate ion, H2NSO3, can be thought of as having been formed from the amide ion, NH2, and sulphur trioxide, SO3. (a) What are the electron-pair and molecular geometries or the amide ion and or SO3? What are the hybridizations of the N and S atoms, respectively? (b) Sketch a structure for the sulfamate ion, and estimate the bond angles. (c) What changes in hybridization do you expect for N and S in the course of the reaction NH2 + SO3 H2NSO3? (d) Is SO3 the donor of an electron pair or the acceptor of an electron pair in the reaction with amide ion? Does the electrostatic potential map shown below confirm your prediction?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





