
Classify each alcohol as 1°, 2°, or 3o
a.
b.
(a)
Interpretation:
The following alcohol should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
Answer to Problem 23P
Primary alcohol
Explanation of Solution
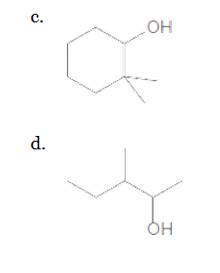
The given compound is
According to the above structure of compound, one hydroxyl group is present on carbon atom which is linked with one other carbon atom. Thus,
(b)
Interpretation:
The following alcohol should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
Answer to Problem 23P
Tertiary alcohol
Explanation of Solution
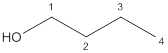
The given compound is
According to the above structure of compound, one hydroxyl group is present on carbon atom which is linked with three other carbon atoms. Thus,
(c)
Interpretation:
The following alcohol should be classified as
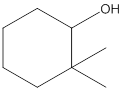
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
Answer to Problem 23P
Secondary alcohol
Explanation of Solution
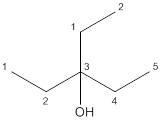
The given structure is:
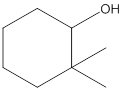
According to the above structure of compound, one hydroxyl group is present on carbon atom which is linked with two other carbon atoms. Thus, the given compound is secondary alcohol.
(d)
Interpretation:
The following alcohol should be classified as
Concept Introduction:
Compounds consist of carbon and hydrogen is known as hydrocarbons. Hydrocarbons are classified as saturated hydrocarbon and unsaturated hydrocarbon. Saturated hydrocarbons are those hydrocarbons in which carbon-carbon single bond is present as carbon is linked with four atoms. Unsaturated hydrocarbons are those hydrocarbons in which carbon-carbon multiple bonds are present that is double and triple bond.
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to one other carbon atom is known as primary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to two other carbon atoms is known as secondary (
When the hydroxyl group (-OH) is present on the carbon atom and that carbon atom is bonded to three other carbon atoms is known as tertiary (
Answer to Problem 23P
Secondary alcohol
Explanation of Solution
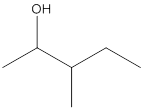
The given structure is:
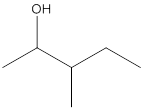
According to the above structure of compound, one hydroxyl group is present on carbon atom which is linked with two other carbon atoms. Thus, the given compound is secondary alcohol.
Want to see more full solutions like this?
Chapter 14 Solutions
GENERAL,ORGANIC,+BIOLOG.CHEM. (LOOSE)
- 1. Which alcohol has a higher boiling point?a. (i) 2-methylpropan-2-ol or (ii) butan-2-olb. (i) hexan-1-ol or (ii) 3,3-dimethylbutan-1-olarrow_forward1. About what volume does 0.15 mol of 2-methylcyclohexanol occupy at room temperature? 15.0 mL 18.5 mL 20.0 mLarrow_forwardGive a systematic (IUPAC) name for each diol.(a) CH3CH(OH)(CH2)4CH(OH)C(CH3)3arrow_forward
- Molecule Type Boiling point (°C) CH3CH2CH3 Alkane -42 CH3CHO Aldehyde +21 CH3CH2OH Alcohol +78 i. Why is the boiling point of the aldehyde greater than that of the alkane?ii. Why is the boiling point of alcohol the highest?iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forwardexplain why methanethiol, CH3SH, has a lower boiling point (6°C) than methanol, CH3OH (65°C), even though methanethiol has a higher molecular weightarrow_forwardIsopropyl alcohol is Select one: a. CH3CH2OH b. CH3CH(OH)CH3 c. CH3CH2CH2OH d. CH3OHarrow_forward
- Classify the following alcohols as primary, secondary, or tertiary: a. b. CH3CH2CH2CH2OH c.arrow_forwardArrange these compounds in order of increasing boiling point. (a) 1-butanol, butane, diethylether (b) hexane, 1-hexanol, dipropyletherarrow_forwardGive a systematic (IUPAC) name for each diol.(a) CH3CH(OH)(CH2)4CH(OH)C(CH3)3 (b) HO¬(CH2)8¬OHarrow_forward
- Predict which member of each group is most soluble in water, and explain the reasons for your predictions. phenol, cyclohexanol, or 4-methylcyclohexanolarrow_forwardWhat kind of solvent ingredients is usually used in the concentrations of 4-10 percent in skin care products and their function is to soften skin cells and to lessen wrinkles? A. Ethly acetate B. Alpha hydroxyl acids C. Phenols and phenol derivatives D. Aliphatic alcoholsarrow_forwardpositive and negative impact of alcohol chemistry in sociteyarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





