
Concept explainers
(a)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (
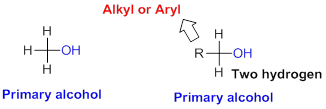
Secondary alcohol:
Hydroxyl group (
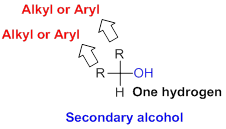
Tertiary alcohol:
Hydroxyl group (
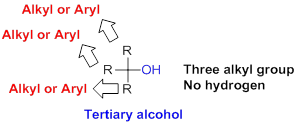
(b)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept Introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (
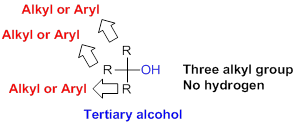
(c)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept Introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(d)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept Introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(e)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(f)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(g)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(h)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

(i)
Interpretation:
The alcohol should be identified as primary, secondary or tertiary alcohol.
Concept introduction:
Classification of alcohol:
Alcohols can be classified in to three types,
Primary,
Secondary,
Tertiary
Primary alcohol:
Hydroxyl group (

Secondary alcohol:
Hydroxyl group (

Tertiary alcohol:
Hydroxyl group (

Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Edition; Modified Mastering Chemistry with Pearson eText -- ValuePack ... and Biological Chemistry (4th Edition)
- Draw the full structure of triglyceride 1-docosahexanoyl-2-arachidonyl-3-elaidyl-glycerol with proper stereochemistry, given the following information: docosahexanoic acid: 22:6n-3, arachidonic acid: 20:4n-6, elaidic acid: trans-18:1n-9,arrow_forwardWhat makes 2, 4-dinitrophenylhydrazine suitable for characterizing aldehydes and ketones? How would carbonyl groups in the two pheromones be distinguished by a suitable chemical method?arrow_forwardThe explosive trinitrotoluene (TNT) is made by carrying out three successive nitration reactions on toluene. If these nitrations only occur in the ortho and para positions relative to the methyl group, what is the structure of TNT?arrow_forward
- What is the ionisable group of phenazopyridine? With illustrationsarrow_forwardThe reaction of methoxy benzene with hydrogen iodide will yield a phenol and an alkyl halide. Which of following choices is the correct combination of the products?arrow_forwardWrite the formulas of the four singly chlorinated isomers formed when 2-methylbutane reacts with Cl2 in the presence of light.arrow_forward
- One of these forms of cocaine is relatively insoluble inwater: which form, the free base or the hydrochloride?arrow_forwardThis is the structure of 2,4-dimethyl-1-pentanol.arrow_forwardHow can chirality and stereoisomers influence the pharmacology, bioactivity, toxicology, pharmacokinetics, and metabolism of ibuprofen? Please provide a detailed summary of what might happen if, for instance, the R enantiomer isn't able to be inverted to the bioactive S enantiomer.arrow_forward
- With respect to the parent compound, explain in detail how modifying the rings shown as A, B, C, D and E in morphine can affect the biological activity associated with the moleculearrow_forwardWhat structural feature is necessary for an alcohol to undergo oxidation reactions?arrow_forwardTriacyl glycerols can be hydrolyzed under basic conditions to give ________ (a 3 carbon compound) and __________.arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





