
Concept explainers
(a)
Interpretation:
The structure of the
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image.
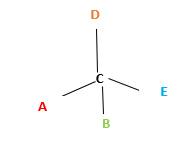
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different
(b)
Interpretation:
The structure of the alcohol of molecular formula
Concept Introduction:
Chirality is the presence of an asymmetric carbon center in a molecule and a molecule which contains a chiral center cannot superimpose on its mirror image.
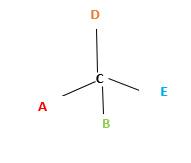
In the above diagram, where C is the chiral center/ asymmetric carbon center.
A, B, D, E are four different functional groups.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
- Draw the structures of the following molecules: (a) Biacetyl, C4H6O2, a substance with the aroma of butter; it contains no rings or carbon-carbon multiple bonds. (b) Ethylenimine, C2H5N, a substance used in the synthesis of melamine polymers; it contains no multiple bonds. (c) Glycerol, C3H8O3, a substance isolated from fat and used in cosmetics; it has an -OH group on each carbon.arrow_forwardArrange these compounds in order of increasing boiling point (values in C are 42, 24, 78, and 118). (a) CH3CH2OH (b) CH3OCH3 (c) CH3CH2CH3 (d) CH3COOHarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





