
Concept explainers
(a)
Interpretation:
How UV-vis spectroscopy could be used to determine whether the given reaction has actually taken place or not is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction will take place as suggested by change in the
Explanation of Solution
The given reaction is:
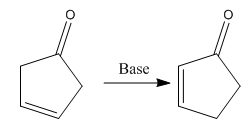
In the above reaction, both the reactant and product have two double bonds; in the product, both double bonds are in conjugation, but in the reactant, they are not.
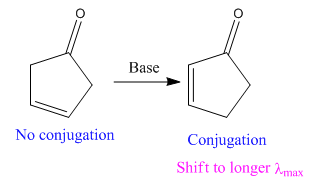
Thus, we can say that the given reaction has actually occurred.
Whether the given reaction has actually taken place is suggested on the basis of conjugation in the product and its effect on
(b)
Interpretation:
How UV-vis. Spectroscopy could be used to determine whether the given reaction has actually taken place or not is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction does not occur as given, since the
Explanation of Solution
The given reaction is:
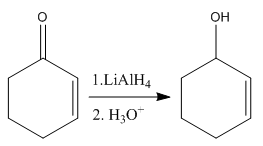
In the above reaction, the reactant has two double bonds in conjugation, and the product has only one double bond. Thus,
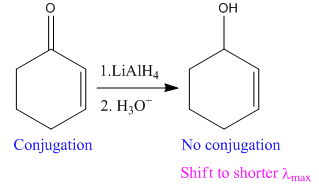
Thus, we can say that the given reaction does not take place.
Whether the given reaction has actually taken place is suggested on the basis of conjugation in the product and its effect on
(c)
Interpretation:
How UV-vis. Spectroscopy could be used to determine whether the given reaction has actually taken place or not is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction not take place as given, since the
Explanation of Solution
The given reaction is:
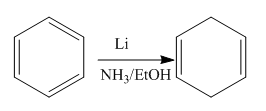
In the above reaction, the reactant has three double bonds in conjugation, and the product has two double bonds not in conjugation. The value of the longest-wavelength
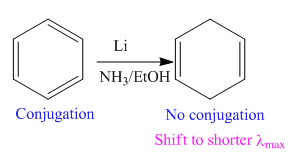
Thus,
Whether the given reaction actually took is suggested on the basis of conjugation in the product and its effect on
(d)
Interpretation:
How UV-vis. Spectroscopy could be used to determine whether the given reaction has actually taken place or not is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction will take place as suggested by change in the
Explanation of Solution
The given reaction is:
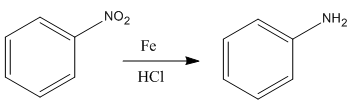
In the above reaction, both reactant and product have three triple bonds in conjugation, but the reactant has a lone pair. So, the reactant’s longest-wavelength absorption corresponds to a
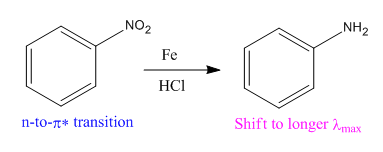
Therefore the
Whether the given reaction actually took is suggested on the basis of conjugation in the product and its effect on
(e)
Interpretation:
How UV-vis. Spectroscopy could be used to determine whether the given reaction actually took is to be suggested with reason.
Concept introduction:
Compounds with lone pairs tend to have longer-wavelength absorptions than analogous compounds without lone pairs. The longest
Answer to Problem 15.36P
The given reaction will take place as suggested by change in the
Explanation of Solution
The given reaction is:
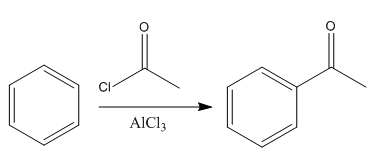
In the above reaction, the reactant has three conjugated bonds, and the product has four conjugated double bonds.
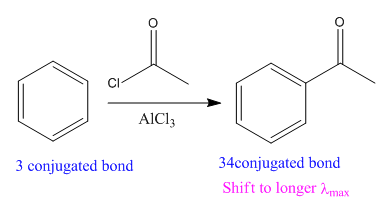
Thus, we can say that the given reaction is actually took place.
Whether the given reaction actually took is suggested on the basis of conjugation in the product and its effect on
Want to see more full solutions like this?
Chapter 15 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- List the four spectroscopy methods that were discussed in class and briefly discuss the principles upon which each one is based (b) Describe how you could use any two of the methods listed in (a) to differentiate between the following two compounds: (i) Butanol and (ii) 2-Butanone.arrow_forwardExplain in detail Remote Spectroscopy via NIRarrow_forwardThis is referring to infrared spectroscopy. Why does the first double bond have a weak/non-existent peak while the second double bond has a moderate intensity peak?arrow_forward
- in the experiment involves Friedel- Crafts acylation. why does the synthesized compound exhibited fluorescence activity. and which layer in the separatory funnel it is found?arrow_forwardWhy acid is added to the solution when analyzing mixtures in the UV-Visible absorption spectrometry.arrow_forwardHow can IR spectroscopy be used to determine when the following reaction is complete?arrow_forward
- For benzanilide, a. find the expected number and ORDER of the 1H NMR signals and the order in which they would appear (most downfield to most upfield). For each signal please give integration value and multiplicity.arrow_forwardYou are doing a ruthenium catalyzed hydrogenation of 4-methylbenzaldehyde (reactant) to 4-methylbenzyl alcohol (product). Draw the reaction equation. Can you use IR spectroscopy to distinguish between reactant and product? Include a detailed list of the characteristic IR bands that you expect to see in your explanation.arrow_forwardHow wouldyou use UV spectroscopy to distinguish between the reactant and the product ?explain with examplearrow_forward
- Based on your interpretation of the mass spectral fragmentation, what are the structures of the two compounds?arrow_forwardWhy are solid forms of the sample are not suitable for Uv-visible spectroscopy and Fluorescence spectroscopy? Please shortly answer at your own words. Answer should be specific (3-4 lines).arrow_forwardUnder what circumstances would you want to use a fluorometric procedure rather than an absorption spectrometry procedure? Briefly explain.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





