
Concept explainers
(a)
Interpretation:
Given the reaction was formed by acetal (or) ketal, to draw the target molecule transformation should be identified.
Concept Introduction:
Acetal formation: The small amount of acid catalysts is added to the reaction of an alcohol with an
A hemiacetal or a hemiketal: addition of alcohol to an aldehyde or ketone which produce hemi acetal.
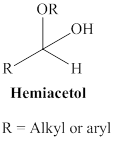
Acetal: An acetal is one of organic molecule where two separate oxygen atoms are single bonded to a central carbon atom it is called acetal. In other words compound that has two ether groups
(b)
Interpretation:
The given hydrochlorination of an alkene should be completed
Concept Introduction:
Hydrohalogenation:
(c)
Interpretation:
The addition reaction should be drawn and identified given the alcohol molecule in presence of acidic condition.
Concept Introduction:
Dehydration reaction:
Removal of water molecule from the reaction when the alcohol is treated with strong acid like sulfuric acid.
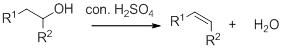
Alcohol is reaction with concentrated sulfuric acid, first alcohol gets protonated forms carbocation (more stable carbocation) followed by elimination of proton (
Tertiary carbocation is more stable than the secondary, secondary carbocation is more stable than primary.

In dehydration reaction, sulfuric acid is act as a proton donor, and which is used to protonate the alcohol and makes carbocation therefore sulfuric acid is the driving force of the reaction. Dehydration reaction will not go without acid (sulfuric acid).
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning



