
Concept explainers
(a)
Interpretation:
A concept map is to be drawn and the grams of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 19E
The concept map is shown below.
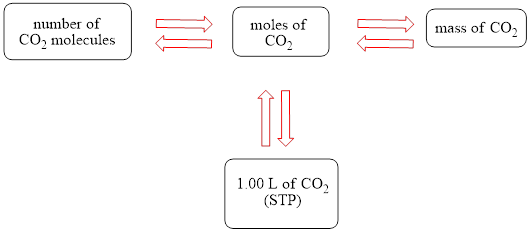
The grams of
Explanation of Solution
When

Figure 1
The volume occupied by
Therefore, the number of moles which occupy
The molar mass of
Therefore, the mass of
The formula to calculate the mass of
Substitute the mass of
Therefore, the grams of
The grams of
(b)
Interpretation:
A concept map is to be drawn and the molecules of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 19E
The concept map is shown below.

The molecules of
Explanation of Solution
When

Figure 1
The volume occupied by
Therefore, the number of moles which occupy
The molecules present in
The formula to calculate the molecules occupied by
Substitute the molecules in
Therefore, the molecules of
The molecules of
(c)
Interpretation:
A concept map is to be drawn and the molar concentration of the carbonic acid solution when
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 19E
The concept map is shown below.

The molar concentration of the carbonic acid solution is
Explanation of Solution
When

Figure 1
The volume occupied by
Therefore, the number of moles which occupy
The number of moles in
The relation between
The probable unit factors are given below.
The unit factor to determine
Therefore, the volume in
The formula to determine molarity is shown below.
Where
•
•
•
Substitute the value of number of moles and volume in equation (1).
The relation between
The unit factors are given below.
The unit factor to determine
Therefore,
Therefore, the molar concentration of
The molar concentration of carbonic acid solution is
Want to see more full solutions like this?
Chapter 15 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- What volume of 0,250 mol⋅dm–3 H2SO4 (sulfuric acid) will react with 42,4 cm3 of 1,50 mol⋅dm-3 Na2CO3 (sodium carbonate)? (Hint: Write the balanced equation first.) 2. This problem is similar to the combustion analysis problem : An unknown compound has the formula CxHyOz. When 0,1523 g of the compound is burned in oxygen, 0,3718 g of CO2 and 0,1522 g of H2O is produced. a) What is the empirical formula of the unknown compound? b) If the molar mass of the unknown compound is 72,1 g/mol, what is its molecular formula?arrow_forwardGiven that 1.00 g of hydrogen fluoride dissolves in 100.0 mL of solution, draw a mole concept map and calculate each of the following. (a) liters of HF gas (STP) dissolved in the solution (b) molecules of HF gas dissolved in the solution (c) molar concentration of the hydrofluoric acid solutionarrow_forwardWhat are the number of moles in one litre of water assume that water density is 1 g/mol.arrow_forward
- Please do the question where it states "using the ideal gas law, calculate the theoretical yield of hydrogen gas!" ****Where you have to find L H2 and mL H2 (theoretical yield)arrow_forwardHow many particles are present in 936 milliliters of hydrogen gas at standard temperature and pressure? to the nearest thousandthsarrow_forwardWhat would be the molecular weight of a gasarrow_forward
- how many moles of gas will be produced if 0.0595 moles reacted completely with acetic acid?arrow_forwardt snowed, it melted and now the temperature is going to dip below freezing, to -2.00 °C overnight. You notice a puddle of water in the driveway of your house. You don’t want to start your morning by slipping on the ice, so you decide to put some salt down before the temperature dips. You guess the puddle contains about 10 L of water. How much salt (NaCl) would you have to throw into the water to prevent it from freezing? Molar mass of NaCl is 58.44 g/mol.arrow_forwardSolid aluminum hydroxide forms when 100mL of 1.25mol/L aqueous aluminum acetate is mixed with 300mL of 2.30mol/L aqueous sodium hydroxide. what is the limiting reactant and what is the mass of solid aluminum that formsarrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

