
Concept explainers
(a)
Interpretation:
A concept map is to be drawn and the liters of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 17E
The concept map is shown below.
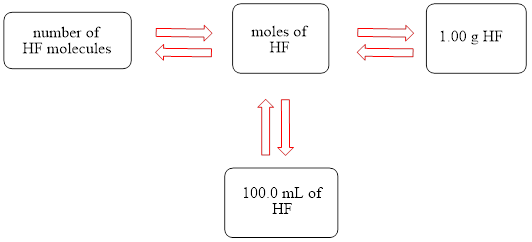
The liters of
Explanation of Solution
When

Figure 1
The formula to calculate the number of moles of
The mass of
The molar mass of
Substitute the mass and molar mass of
The volume occupied by
The formula to calculate the volume occupied by
Substitute the volume of
Therefore, the liters of
The liters of
(b)
Interpretation:
A concept map is to be drawn and the molecules of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 17E
The concept map is shown below.

The molecules of
Explanation of Solution
When

Figure 1
The formula to calculate the number of moles of
The mass of
The molar mass of
Substitute the mass and molar mass of
The molecules present in
The formula to calculate the molecules occupied by
Substitute the molecules in
Therefore, the molecules of
The molecules of
(c)
Interpretation:
A concept map is to be drawn and molar concentration of the hydrofluoric solution in the solution in when
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 17E
The concept map is shown below.

The molar concentration of the hydrofluoric acid solution is
Explanation of Solution
When

Figure 1
The formula to calculate the number of moles of
The mass of
The molar mass of
Substitute the mass and molar mass of
The number of moles in
The relation between
The probable unit factors are given below.
The unit factor to determine
Therefore, the volume in
The formula to determine molarity is shown below.
Where
•
•
•
Substitute the value of number of moles and volume in equation (1).
The relation between
The unit factors are given below.
The unit factor to determine
Therefore,
Therefore, the molar concentration of
The molar concentration of
Want to see more full solutions like this?
Chapter 15 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- Classify each of the following solutes into the approximate solubility categories of Table 7.3. The numbers in parentheses are the grams of solute that will dissolve in 100g of water at temperature indicated. a.barium nitrate, Ba(NO3)2(8.7gat20C) b.aluminum oxide, Al2O3(9.8105gat29C) c.calcium sulfate, CaSO4(0.21gat30C) d.manganese chloride, MnCl2(72.3gat25C) e.lead bromide, PbBr2(0.46gat0C)arrow_forwardThe hard water scum that forms a ring around the bathtub is an insoluble soap Ca(C18H35O2)2. It is formed when a soluble soap, NaC18H35O2, reacts with the calcium ion that is responsible for the hardness in water:2NaC18H35O2+Ca2+Ca(C18H35O2)2+2Na+. How many milligrams of scum can form from 616mg of NaC18H35O2? Hard water contains calcium ion, which reacts with the stearate ion in soap to form bathtub scum.arrow_forwardPotassium hydroxide is used in making liquid soap. How many grams would you use to prepare 2.50L of 1.40M potassium hydroxide?arrow_forward
- What is first required before solving a stoichiometry problem?arrow_forward! (Solve the following problem and give the correct answer with COMPLETE UNIT. Solution must also include the BALANCED chemical equation for the problem. (use 1 decimal place in atomic weight)arrow_forwardTopic: stoichiometry Convert the following from moles to grams. Write your solution.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


