
Concept explainers
(a)
Interpretation:
A concept map is to be drawn and the grams of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 16E
The concept map is shown below.
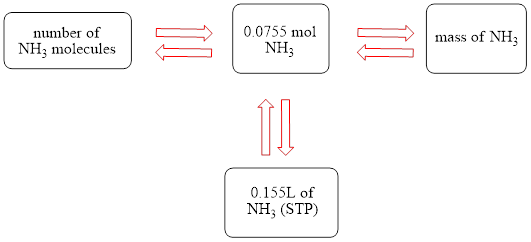
The grams of
Explanation of Solution
When

Figure 1
The molar mass of
Therefore, the mass of
The formula to calculate the mass of
Substitute the mass of
Therefore, the grams of
The grams of
(b)
Interpretation:
A concept map is to be drawn and the liters of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 16E
The concept map is shown below.

The liters of
Explanation of Solution
When

Figure 1
The volume occupied by
The formula to calculate the volume occupied by
Substitute the volume of
Therefore, the liters of
The liters of
(c)
Interpretation:
A concept map is to be drawn and the molecules of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 16E
The concept map is shown below.

The molecules of
Explanation of Solution
When

Figure 1
The molecules present in
The formula to calculate the molecules occupied by
Substitute the molecules in
Therefore, the molecules of
The molecules of
(d)
Interpretation:
A concept map is to be drawn and the molar concentration of the ammonia solution when
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 16E
The concept map is shown below.

The molar concentration of the ammonia solution is
Explanation of Solution
When

Figure 1
The number of moles in
The formula to determine molarity is shown below.
Where
•
•
•
Substitute the value of number of moles and volume in equation (1).
The relation between
The unit factors are given below.
The unit factor to determine
Therefore,
Therefore, the molar concentration of
The molar concentration of
Want to see more full solutions like this?
Chapter 15 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- What is the total number of moles of gas produced from the decomposition 13.0 g of guanidinium nitrate?arrow_forwardIn gas stoichiometry problems, what is used as the bridge between amount in moles and volume?arrow_forwardGiven: 0.05056kg of NH4CL Question: Determine the moles of NH4Cl in the sample.arrow_forward
- The hard water scum that forms a ring around the bathtub is an insoluble soap Ca(C18H35O2)2. It is formed when a soluble soap, NaC18H35O2, reacts with the calcium ion that is responsible for the hardness in water:2NaC18H35O2+Ca2+Ca(C18H35O2)2+2Na+. How many milligrams of scum can form from 616mg of NaC18H35O2? Hard water contains calcium ion, which reacts with the stearate ion in soap to form bathtub scum.arrow_forwardWhat is the theoretical/calculated gram mass for silver metal? (only need to report number below)arrow_forwardHow many liters of carbon dioxide will react with sodium hydroxide solution that has a mass of 60.0grams?Please solve with the given formulaarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


