
Concept explainers
(a)
Interpretation:
A mole concept map is to be drawn and the grams of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 20E
The mole concept map is shown below.
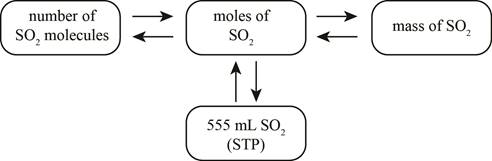
The grams of
Explanation of Solution
When
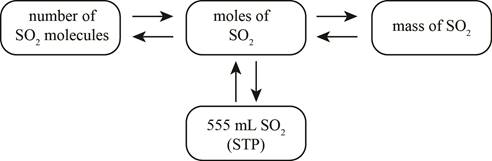
Figure 1
The volume occupied by
The volume of dissolved
The relation between
The probable unit factors are given below.
The unit factor to determine
Therefore, the volume in
Therefore, the number of moles which occupy
The molar mass of
Therefore, the mass of
The formula to calculate the mass of
Substitute the mass of
Therefore, the grams of
The grams of
(b)
Interpretation:
A mole concept map is to be drawn and the molecules of
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 20E
The mole concept map is shown below.
The molecules of
Explanation of Solution
When
Figure 1
The volume occupied by
The volume of dissolved
The relation between
The probable unit factors are given below.
The unit factor to determine
Therefore, the volume in
Therefore, the number of moles which occupy
The molecules present in
The formula to calculate the molecules occupied by
Substitute the molecules in
Therefore, the molecules of
The molecules of
(c)
Interpretation:
A mole concept map is to be drawn and the molar concentration of the sulfuric acid solution when
Concept introduction:
A mole is a basic unit used in the International system of units (SI). It is abbreviated as
Answer to Problem 20E
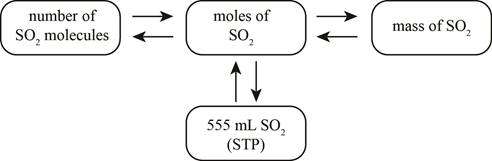
The mole concept map is shown below.
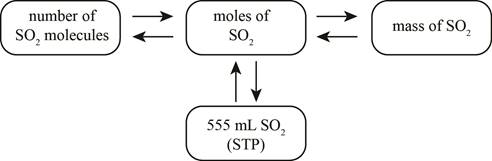
The molar concentration of the sulfuric acid solution is
Explanation of Solution
When
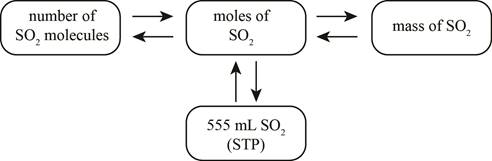
Figure 1
The volume occupied by
The volume of dissolved
The relation between
The probable unit factors are given below.
The unit factor to determine
Therefore, the volume in
Therefore, the number of moles which occupy
The number of moles in
The formula to determine molarity is shown below.
Where
•
•
•
Substitute the value of number of moles and volume in equation (1).
The relation between
The unit factors are given below.
The unit factor to determine
Therefore,
Therefore, the molar concentration of
The molar concentration of sulfurous acid solution is
Want to see more full solutions like this?
Chapter 15 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- What is volume occupied by mixture of 2g hydrogen, 8g of oxygen and 14 g of nitrogen?arrow_forwardWhat is the value for the molar volume of a gas at STParrow_forwardBalance SO2(g) + O2(g) → SO3(g) given 2.4 mol of O2 and then determine the volume of the indicated reactant at STP that is required for complete reaction. Assuming complete reaction, what is the volume of the products?arrow_forward
- Given that 1.00 g of hydrogen fluoride dissolves in 100.0 mL of solution, draw a mole concept map and calculate each of the following. (a) liters of HF gas (STP) dissolved in the solution (b) molecules of HF gas dissolved in the solution (c) molar concentration of the hydrofluoric acid solutionarrow_forwardWhat would be the molecular weight of a gasarrow_forwardHow many liters of oxygen gas are required to completely react with 2.4L of hydrogen gas to form waterarrow_forward
- Suppose instead that the original cylinders contained ½ mol of O2 (g) and 3/2 mol of N2 (g), respectively, each at room temperature and atmospheric pressure. The gases are then mixed at room temperature and allowed to expand to equilibrium against atmospheric pressure. The final volume of the gas mixture.arrow_forwardWrite Van der waal’s equation for n moles of a gas.arrow_forwardA gas that is produced when fruit ripens contain 85.62% C and 14.38% H by mass. The molecular mass was found to be twice the empirical formula unit molecular mass. What is the molecular formula of this gas?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
