
(a)
Interpretation:
The stoichiometry concept map is to be drawn and the mass of reacted methane
Concept introduction:
Stoichiometry measures the amount of reactant and product consumed or formed in a
Answer to Problem 23E
The stoichiometry concept map is shown below.
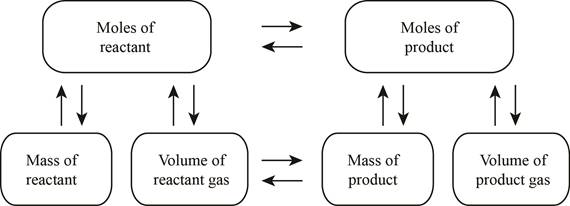
The mass of the reacted methane is
Explanation of Solution
It is given that
The stoichiometry concept map is shown below.
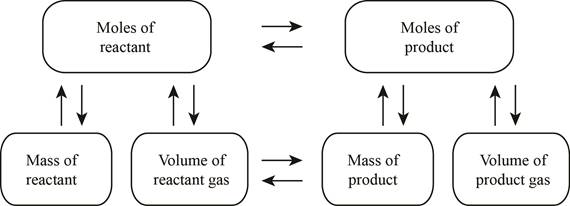
Figure 1
The chemical reaction (1) should be balanced first to calculate the mass of reacted methane.
The number of oxygen atoms is not equal on both sides. A coefficient of
The number of hydrogen atoms is not equal on both sides. A coefficient of
The balanced equation is shown below.
The mass of methane reacted is equal to the mass of
The molar mass of hydrogen is
The molar mass of oxygen is
Therefore, the number of moles of methane is calculated from the number of moles of water using a stoichiometric ratio as shown below.
For
The mass of methane reacted can be calculated from the number of moles of methane which is
The molar mass of carbon is
The molar mass of hydrogen is
The number of moles of methane can be calculated from the relation shown below.
The mass of methane for
Therefore, the mass of methane is
The stoichiometry concept map is shown in Figure 1. The mass of methane consumed in the reaction is
(b)
Interpretation:
The volume of carbon dioxide
Concept introduction:
Stoichiometry measures the amount of reactant and product consumed or formed in a chemical reaction. It gives the relationship between the reactants and products. Stoichiometry concept maps are used to determine the relation of reactant and product in terms of stoichiometry.
Answer to Problem 23E
The stoichiometry map is shown below.
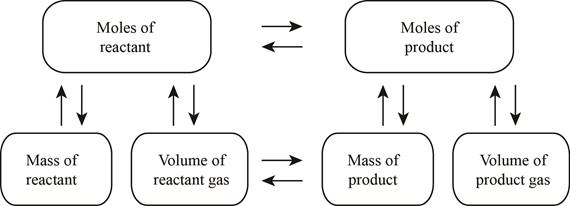
The volume of carbon dioxide produced at
Explanation of Solution
The stoichiometry concept mass is shown below.
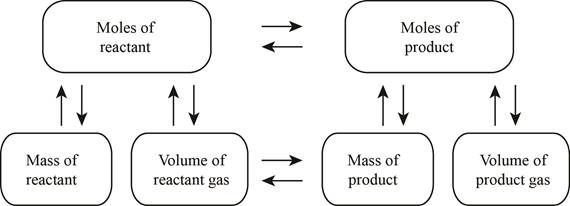
Figure 1
The balanced chemical reaction is stated in part (a) named as equation (2) is used to calculate the volume of
The equation (2) shows that 1 mole of methane is consumed to form 1mole of carbon dioxide.
Therefore, the volume for
Therefore, the volume of
The volume of
Want to see more full solutions like this?
Chapter 15 Solutions
INTRODUCTORY CHEMISTRY-STD.GDE.+SOL.MAN
- Barium Hydroxide Octahydrate and Ammonium Chloride react to form Barium Chloride, Water and Ammonia (NHs). If 150.00g of Barium Hydroxide Octahydrate react with excess Ammonium Chloride, how many molecules of ammonia will be produced?arrow_forward___________ to ____________ 2KClO3 → 2KCl + 3O2 Given Units Unknown Units How many liters of oxygen are produced if 346 grams of KClO3 decomposes @ STP?arrow_forwardThe mass of the plastic bag, baking soda, and vinegar before the reaction was equal to the mass of the plastic bag, baking soda, and vinegar after the reactionBubbles were produced during the reaction which was evidence a gas was being producedThe plastic bag did not change in any way which indicated it was not involved in the reactionThe mass of the baking soda was exactly equal to the mass of the vinegar used to create the reaction.arrow_forward
- Water breaks down to form hydrogen and oxygen. How many liters of oxygen are producedwhen 75.0g of water decomposes?arrow_forwardWhat volume of water is produced when a mixture of 150cm³ of hydrogen and 100cm³ of oxygen is exploded in a eudiometerarrow_forwardWhat volume of carbon dioxide gas (in mL) is produced when 346 mL of acetylene gas undergoes complete combustion? Assume all reactant and product volumes are measured at SATP.arrow_forward
- In a reaction between 0.8255g of calcium chloride dihydrate and 5ml of 5M sodium carbonate, what is the number of moles of calcium carbonate produced? What is the theoritical mass of calcium carbonate produced?arrow_forwardIron(III) chloride is used to treat drinking and waste water. It can be made by reacting iron with chlorine gas. Calculate the volume of chlorine gas (Cl2) STP that will react with 5.00 grams of ironarrow_forwardNitric acid is produced fromnitrogen monoxide, which is in turnis prepared from ammonia by a process known as the Ostwald process, shown below. If 250. mL of oxygen reactswith 250. mL of ammonia, what mass(in g)of nitrogen monoxide will be producedat STP?arrow_forward
- 6.The combustion of propane gas produces carbon dioxide and water vapor.C3H8(g)+5O2(g)→3CO2(g)+4H2O(g)What volume of oxygen is required to completely combust 0.650L of propane? What volume of carbon dioxide is produced in the reaction? (molar volume ratio is treated similarly like molar ratio)arrow_forwardA scale model of a blimp rises when it is filled with helium to a volume of 55.0 dm³. When 1.10 mol of He is added to the blimp, the volume is 26.2 dm³. How many more grams of He must be added to make it rise? Assume constant Tand P.arrow_forwardGrease fires can be extinguished by applying baking soda (sodium hydrogen carbonate), which decomposes due to the heat to produce sodium oxide, carbon dioxide and water. The carbon diocide produced helps to smother the flames. How many grams of baking soda would need to be thrown on to a fire to produce 0.500 moles (approximately 11 L) of carbon dioxide gas?arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co



