
(a)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
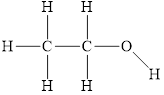
Figure 1
The molecule has electronegative atom
The intermolecular interactions that operate in
(b)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
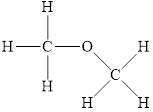
Figure 2
The molecule has electronegative atom
The intermolecular interactions that operate in
(c)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
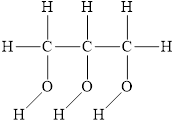
Figure 3
The molecule has electronegative atom
The intermolecular interactions that operate in
(d)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
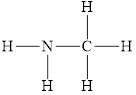
Figure 4
The molecule has electronegative atom
The intermolecular interactions that operate in
(e)
Interpretation:
The intermolecular interactions that operate in
Concept introduction:
There are three types of interactions through which the molecules are stabilized. They are hydrogen-bonding interactions, the dipole-dipole interactions and the induced dipole interactions. These interactions influence the properties of the compounds like boiling point, melting point and so on.
Answer to Problem 39E
The intermolecular interactions that operate in
Explanation of Solution
In all molecular substances, the intermolecular interactions exist. These interactions are induced dipole interactions, dipole-dipole interactions and hydrogen bonding.
The structure of
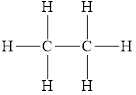
Figure 5
The molecule does not have any has electronegative atom. This means the polarity is not present in the molecule. This molecule can form bond with other molecule through the induced dipole interactions. Therefore, the dominant intermolecular interaction in this molecule is induced dipole interaction.
The intermolecular interactions that operate in
Want to see more full solutions like this?
Chapter 15 Solutions
Bundle: Introductory Chemistry: An Active Learning Approach, 6th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- What is the energy change when the temperature of 10.1g of solid silver is decreased from 38.1C to 23.6C?arrow_forwardThe covalent compounds ethyl alcohol and dimethyl ether both have the formula C2H6O. However, the alcohol melts at 117.3C and boils at 78.5C, whereas the ether melts at 138.5C and boils at 23.7C. How could differences in forces between molecules be used to explain these observations?arrow_forwardThe forces that are bond between ions in the ionic solid are described as a) metalic forces b) London dispersion forces c) Electrostatic attractions forcesarrow_forward
- True or false. When a liquid is placed inside a capillary and the cohesive forces are atronger than the adhesive forces, the meniscus formed is convex.arrow_forwardSelect all of the intermolecular forces that are present in dimethylamine. A.)London Dispersion Forces B.)Dipole-Dipole Forces C.)Hydrogen Bondingarrow_forwardWhich of the following intermolecular forces would be present between molecules ofsulfur tetrafluoride and water?A.) DispersionB.) Dipole-DipoleC.) Hydrogen-BondingD.) Ion-Dipolearrow_forward
- Phosphorus trichloride is polar. Which intermolecular forces are present? a. dipole-dipole forces only b. London dispersion forces and dipole-dipole forces c. London dispersion forces and dipole-dipole forces and hydrogen bonding d. hydrogen bonding only e. London dispersion forces onlyarrow_forwardWhat is the density of vapor in flaskarrow_forwardWhich of the following forces results from a temporary dipole (instantaneous dipole)? Group of answer choices Dispersion forces Hydrogen bonding Ion-dipole forces Covalent bonding Dipole-dipole forces need in detail explaain y other options are incorrect also or else I will downvotearrow_forward
- Identifying the Type of Intermolecular Forces. Identify which is the most important type of "intermolecular" force for each of the following:arrow_forwardThe following compounds are liquids at room temperature. Which among these compounds is the MOST viscous? refer to picarrow_forwardIntermolecular forces are forces that exist ____________ molecules, while intramolecular forces areforces that exist ____________ molecules. a. to condense, to expand b. within; between c. between; within d. to saturate, to unsaturate e. tovibrate, to rotatearrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



