
(a)
Interpretation:
The diagram that represents the
Concept introduction:
Nuclear spin shows the complete
Answer to Problem 16.43E
The diagram that represents the
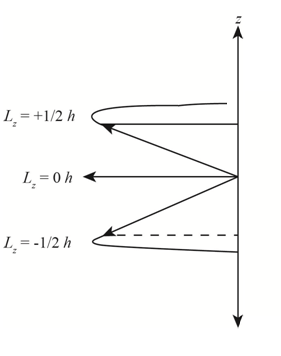
Explanation of Solution
The
Where,
•
The value of
The value of
Substitute value of
Substitute value of
Substitute value of
Therefore, the
The diagram that represents the

Figure 1
The diagram that represents the
(b)
Interpretation:
The diagram that represents the
Concept introduction:
Nuclear spin shows the complete angular momentum corresponding to the nucleus. It is represented by
Answer to Problem 16.43E
The diagram that represents the
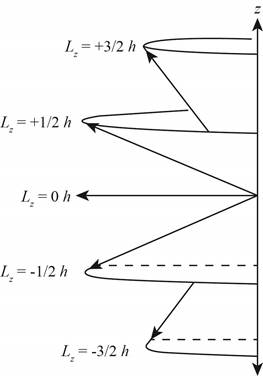
Explanation of Solution
The
Where,
•
The value of
The value of
Substitute value of
Substitute value of
Substitute value of
Substitute value of
Substitute value of
Therefore, the
The diagram that represents the
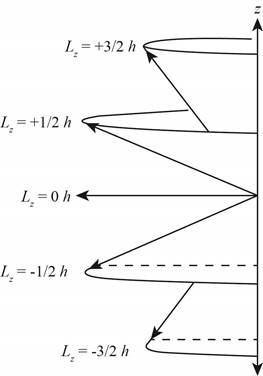
Figure 2
The diagram that represents the
(c)
Interpretation:
The diagram that represents the
Concept introduction:
Nuclear spin shows the complete angular momentum corresponding to the nucleus. It is represented by
Answer to Problem 16.43E
The diagram that represents the
Explanation of Solution
The
Where,
•
The value of
The value of
Substitute value of
Therefore, the
The diagram that represents the
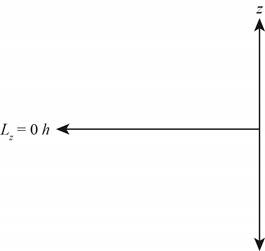
Figure 3
The diagram that represents the
(d)
Interpretation:
The diagram that represents the
Concept introduction:
Nuclear spin is the total angular momentum of the nucleus. It is represented by
Answer to Problem 16.43E
The diagram that represents the
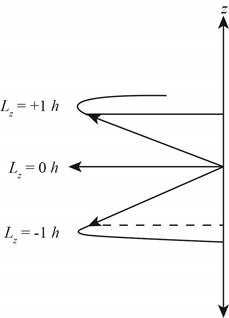
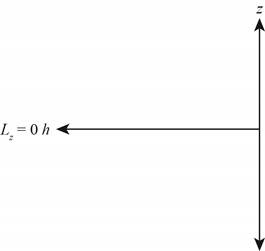
Explanation of Solution
The
Where,
•
The value of
The value of
Substitute value of
Substitute value of
Substitute value of
Therefore, the
The diagram that represents the
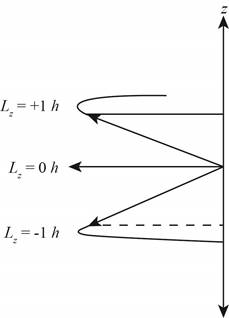
Figure 4
The diagram that represents the
Want to see more full solutions like this?
Chapter 16 Solutions
Physical Chemistry
- The following are sets of rotational quantum numbers (J,MJ,K). Label each indicated transition as either allowed or forbidden. Hint: Remember the rules for allowed values of the various quatum numbers. a (0,0,0)(1,1,0)b (0,0,0)(1,0,0) c (3,2,1)(3,1,1)d (4,4,1)(2,4,1)arrow_forwardIs the bond length in 1HCl the same as that in 2HCl? The wavenumbers of the J = 1 ← 0 rotational transitions for 1H35Cl and 2H35Cl are 20.8784 and 10.7840 cm–1, respectively. Accurate atomic masses are 1.007 825mu and 2.0140mu for 1H and 2H, respectively. The mass of 35Cl is 34.968 85mu. Based on this information alone, can you conclude that the bond lengths are the same or different in the two molecules?arrow_forwardThe nuclear spin quantum number of 14N is 1 and its g-factor is 0.404. Calculate (in joules) the energies of the nuclear spin states in a magnetic field of 10.50 T.arrow_forward
- 8C.4 (a) the moment of inertia of a CH4 molecule is 5.27 x 10^-47 kg m^2. What is the minimum energy needed to start it rotating? 8C.5 (a) use the data in 8C.4 (a) to calculate the energy needed excite a CH4 molecule from a state with l=1 to a state with l=2arrow_forwardA space probe was designed to see 13CO in the atmosphere of Saturn by looking for lines in its rotational spectrum. If the bond length of CO is 112.8 pm, at what wave number do the first three rotational transitions appear?arrow_forwardCalculate the value of ml for a proton constrained to rotate in a circle of radius 100 pm around a fixed point given that the rotational energy is equal to the classical average energy at 25 degrees C.arrow_forward
- : A space probe was designed to seek carbon monoxide in Saturn’s atmosphere by looking for lines in its rotational spectrum. If the bond length of CO is 112.8 pm, at what wavenumbers (in cm-1) do the first three rotational transitions appear? Carbon is almost all carbon-12, so for this part, you can assume it’s all 12C). What resolution would be required to determine the isotropic ratio of 13C to 12C on Saturn by observing the first three 13CO rotational lines as well? (In other words how far apart, in cm-1, are the rotational transitions of 12CO and 13COarrow_forwardYou know that the wavenumbers of the rotational transition J = 1← 0 for 1H35Cl and2H35Cl are 20.8784 and 10.7840cm-1, respectively. Accurate atomic masses are1.007825 and 2.0140 for 1H and 2H, respectively. The mass of 35Cl is34.96885. Conclude based on this information that the bond lengths are the sameor differentarrow_forward3 The rotation motion of HCl molecules can be analyzed by treating each molecule as a rigid rotational constant B= 2.07x10^-22 Joules. a) what frequency of radiation will excite the transition from the J=1 energy level to the J=2 level in this case? b) what is the relative probability of observing an HCl molecule at the J=2 energy level, as compared to teh J=1 level, at 25.00 degrees C?arrow_forward
- The wavenumber for the first Balmer line for Li2+is 136,800 cm-1, what is the wavenumber of the first line of the Balmer series of hydrogen atom? What will be the energy change and frequency of the transition?arrow_forwardWhich is higher in energy: electromagnetic radiation with wavenumber 100 cm−1 or with wavenumber 2000 cm−1?arrow_forward4. A hydrogen atom is in an excited 4f state in an external magnetic field |B| = 1.75 T. Ignoring magnetic spin effects (i.e., ignoring just the effect of ms): what is the difference in energy between the greatest possible energy and least possible energy of the atom?arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
