
(a)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
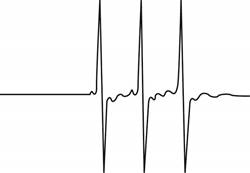
Figure 1
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Three different peaks with same intensity indicate that
Substitute the values of
The above equation is further simplified for the value of
Therefore, the spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
(b)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
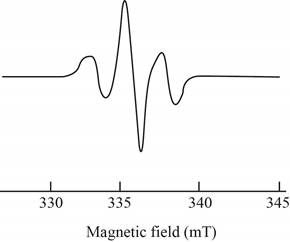
Figure 2
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Three peaks with different intensities indicate that two equivalent nuclei are present.
Substitute the values of
The above equation is further simplified for the value of
Therefore, spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
(c)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
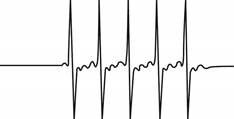
Figure 3
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Five different peaks with same intensity represents that
Substitute the values of
The above equation is further simplified for the value of
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
(d)
Interpretation:
The spin and number of equivalent nuclei required to produce the given ESR spectrum are to be stated.
Concept introduction:
Electron spin resonance is the method to study the materials with an unpaired electron. An unpaired electron absorbs the microwave radiation under the strong magnetic field. The difference between two energy levels is calculated by the formula shown below.
Answer to Problem 16.22E
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Explanation of Solution
The give ESR spectrum is shown below.
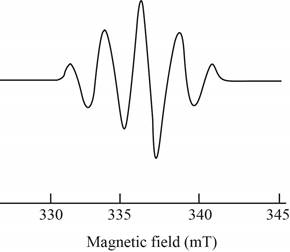
Figure 4
The number of spins in the ESR spectrum is shown below.
Where,
•
•
The total number of signal in the given ESR spectrum is
Five different peaks with different intensities indicate that four equivalent nuclei are present.
Substitute the values of
The above equation is further simplified for the value of
Therefore, the spin and number of equivalent nuclei required to produce the given ESR spectrum are
The spin and number of equivalent nuclei required to produce the given ESR spectrum are
Want to see more full solutions like this?
Chapter 16 Solutions
Physical Chemistry
- Which of the following statements regarding NMR spectroscopy is incorrect?arrow_forwardThis is referring to infrared spectroscopy. Why does the first double bond have a weak/non-existent peak while the second double bond has a moderate intensity peak?arrow_forwardDetermine the structure of the molecule, provide the missing splitting pattern, and label which hydrogens correspond to which chemical shifts.arrow_forward
- Determine the structure of the molecule, provide the missing splitting pattern, and label which hydrogens corresponds to which chemical shifts.arrow_forwardIf the absorption peak(s) was present in the IR spectrum, fill in the approximate wavenumber(s). If the absorption peak(s) was absent in the IR spectrum, write “absent”.arrow_forwardPredict the spin-spin coupling (aka splitting) by providing the multiplicity (singlet, doublet, triplet, doublets of doublet, quartet. etc.) of Ha, Hb, Hc, and Hd of the molecules below. Please decide how many non-equivalent protons in each given molecule.arrow_forward
- Based upon the following NMR SPectrum please list the chemical shifts, multiplicity, and assignments of the major peaksarrow_forwardSpin-spin coupling between two hydrogen nuclei is mediated through chemical bonds. In the ¹H NMR, this coupling is usually observed through neighboring C-H bonds…. Does this means 1 bond???arrow_forwardwhich structure is consistent with the hNMR spectrumarrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



