
Concept explainers
Interpretation:
It should be determined that whether the given substance reacts with aqueous hydrochloric acid or not. If it reacts with
Concept introduction:
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
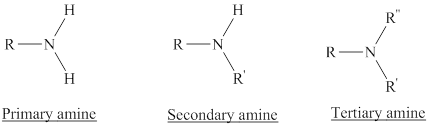
Reaction of amines and acid will give amine salt (ammonium ion).
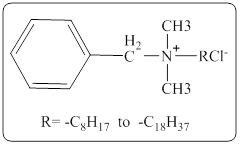
In a quaternary ammonium ion a nitrogen atom with four attached groups is positively charged. And their compounds are known as quaternary ammonium salt.
One commonly encountered quaternary ammonium salt has the following structure,
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Fundamentals of General, Organic, and Biological Chemistry, Books a la Carte Edition (8th Edition)
- The reaction of methoxy benzene with hydrogen iodide will yield a phenol and an alkyl halide. Which of following choices is the correct combination of the products?arrow_forwardOne of these forms of cocaine is relatively insoluble inwater: which form, the free base or the hydrochloride?arrow_forwardThe liquids butan-1-ol and butanal have similar molar masses. Which is expected to have the higher boiling point? Explain your choices.arrow_forward
- Trehalose, a disaccharide found in the blood of insects, has the following structure. What simple sugars would you obtain on hydrolysis of trehalose?arrow_forwardThe structure for 2-chloro-3-heptene is:arrow_forwardWhy do you think compounds containing the heavy metal lead are poisonous?arrow_forward
