
Concept explainers
(a)
Interpretation: The diene and dienophile that are needed to prepare given product is to be stated.
Concept introduction: Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Answer to Problem 16.51P
The diene and dienophile that are needed to prepare given product is shown below.
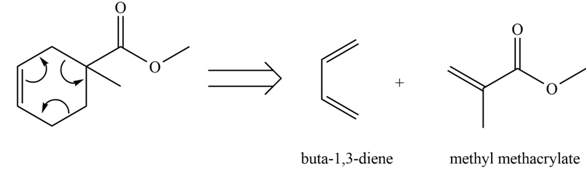
Explanation of Solution
The given product is shown below.
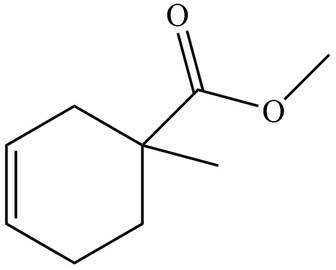
Figure 1
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
Therefore, the diene and dienophile that are needed to prepare given product is shown below.
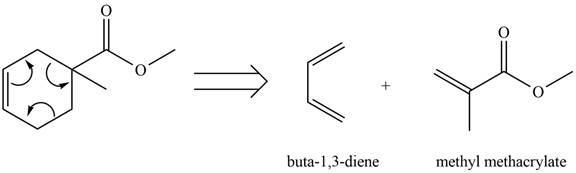
Figure 2
The diene and dienophile that are needed to prepare given product are shown in Figure 2.
(b)
Interpretation: The diene and dienophile that are needed to prepare given product is to be stated.
Concept introduction: Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Answer to Problem 16.51P
The diene and dienophile that are needed to prepare given product is shown below.
Explanation of Solution
The given product is shown below.
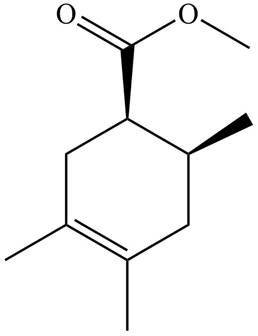
Figure 3
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
Therefore, the diene and dienophile that are needed to prepare given product is shown below.
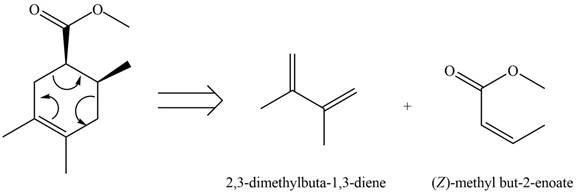
Figure 4
The diene and dienophile that are needed to prepare given product are shown in Figure 4.
(c)
Interpretation: The diene and dienophile that are needed to prepare given product is to be stated.
Concept introduction: Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Answer to Problem 16.51P
The diene and dienophile that are needed to prepare given product is shown below.
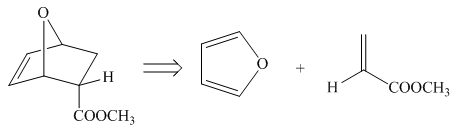
Explanation of Solution
The given product is shown below.
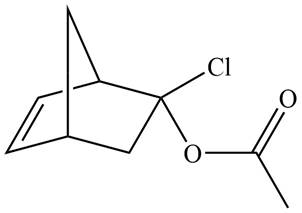
Figure 5
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
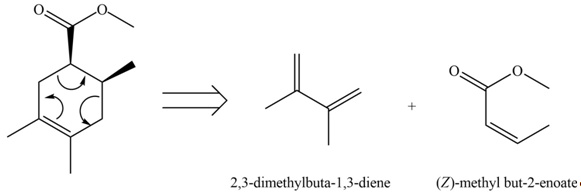
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
Therefore, the diene and dienophile that are needed to prepare given product is shown below.
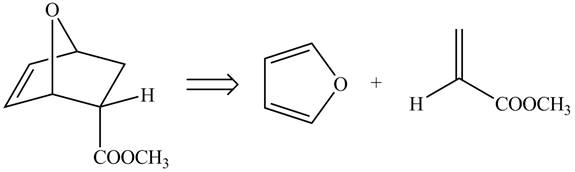
Figure 6
The diene and dienophile that are needed to prepare given product are shown in Figure 6.
(d)
Interpretation: The diene and dienophile that are needed to prepare given product is to be stated.
Concept introduction: Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Answer to Problem 16.51P
The diene and dienophile that are needed to prepare given product is shown below.
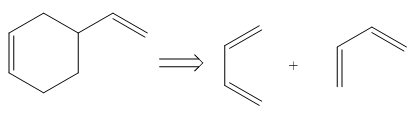
Explanation of Solution
The given product is shown below.
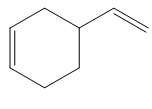
Figure 7
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
Therefore, the diene and dienophile that are needed to prepare given product is shown below.
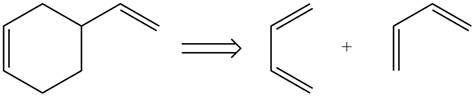
Figure 8
The diene and dienophile that are needed to prepare given product are shown in Figure 8.
(e)
Interpretation: The diene and dienophile that are needed to prepare given product is to be stated.
Concept introduction: Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Answer to Problem 16.51P
The diene and dienophile that are needed to prepare given product is shown below.
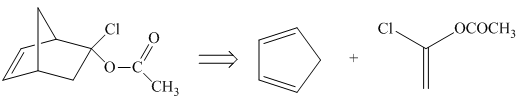
Explanation of Solution
The given product is shown below.
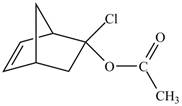
Figure 9
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
Therefore, the diene and dienophile that are needed to prepare given product is shown below.
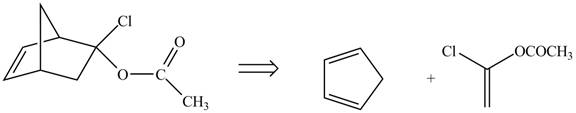
Figure 10
The diene and dienophile that are needed to prepare given product are shown in Figure 10.
(f)
Interpretation: The diene and dienophile that are needed to prepare given product is to be stated.
Concept introduction: Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Answer to Problem 16.51P
The diene and dienophile that are needed to prepare given product is shown below.
Explanation of Solution
The given product is shown below.
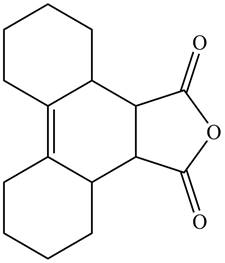
Figure 11
Diels-alder reaction is a cycloaddition reaction in which two molecules combine to form a new ring. In this type of reaction, syn addition takes place. It is a reaction between diene with a dienophile to yield a cyclohexene. The dienophile adds to one side of the diene, and diene adds to the one side of the dienophile. Thus, they have syn stereochemistry.
Retro synthesis can be carried out to identify the diene and dienophile for the given product.
Therefore, the diene and dienophile that are needed to prepare given product is shown below.
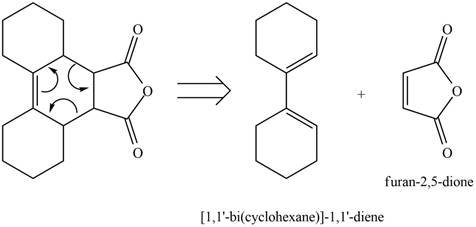
Figure 12
The diene and dienophile that are needed to prepare given product are shown in Figure 12.
Want to see more full solutions like this?
Chapter 16 Solutions
Organic Chemistry - Access (Custom)
- Write down the reduction reaction of each molecule using appropriate reagents, listing the follo compounds according to their of reductionarrow_forwarda.Draw all reasonable resonance structures for pyrrole, and explain why pyrrole is less resonance stabilized than benzene. b.Draw all reasonable resonance structures for furan, and explain why furan is less resonance stabilized than pyrrole.arrow_forwardDraw the product (A) of the following Diels–Alder reaction. A was a key intermediate in the synthesis of the addicting pain reliever morphine, the chapter-opening molecule.arrow_forward
- What are the most likely products of a reaction between CH3CH2NH3 and H2O? a. CH3CH2NH3++OH b. (CH3)2NH2++OH c. CH3CH2NH2OH+H+ d. (CH2)NH2+H3O+arrow_forward(plz with detaol explanation )arrow_forwardThe purine heterocycle occurs commonly in the structure of DNA.a.How is each N atom hybridized? b.In what type of orbital does each lone pair on a N atom reside? c.How many π electrons does purine contain? d.Why is purine aromatic?arrow_forward
- help ! a. What is the relationship between (+)-neomenthol and (-)-menthol? b. Assign absolute stereochemistry (r/s) to each of the stereocenters in (+)-menthol and (-)-menthol (DRAW) c.. Draw the structures of all of menthol's stereoisomerz .. THanks..arrow_forwardDevise a stepwise synthesis of attached compound from dicyclopentadiene using a Diels–Alder reaction as one step. You may also use organic compounds having ≤ 4 C's, and any required organic or inorganicreagents.arrow_forwarddraw compoumds a and barrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

