
Set Up: For an engine, W and QH positive and QC is negative. For a refrigerator, QC is positive and W and QH are negative. A heat pump air conditioner takes in heat at a cool place and expels heat into a warm place. A heat pump house heater takes in heat at a cool place and expels heat into a warm place. For both types of heat pumps, W is negative and energy must be supplied to operate the device, bc and da are adiabatic, so the heat flow for these processes is zero.
Solve: (a) The cycle is clockwise. More positive work is done during ab and bc than the magnitude of the negative work done in cd and da, so the net work done in the cycle is positive. Heat enters the gas in ab and leaves the gas in cd.
(b) The cycle is counterclockwise and the net work done in the cycle is negative. Heat enters the gas in dc and leaves the gas in ba. dc occurs inside the food compartment and ba occurs in the air of the room.
(c) The cycle is counterclockwise and the net work done w the cycle is negative. Heat enters the gas in dc and leaves the gas in ba. dc occurs inside the house and ba occurs outside.
(d) The cycle is counterclockwise and the net work done in the cycle is negative. Heat enters the gas in dc and leaves the gas in ba. dc occurs outside and ba occurs inside the house.
Reflect: For a refrigerator the signs of W, QH, and QC are all opposite to what they are for an engine.
21. The pV diagram in Figure 16.17 shows a general Carnot cycle for an engine, with the hot and cold thermal reservoir segments identified. Segments ab and cd are isothermal, while the other two are adiabatic. (a) If this engine is used as a
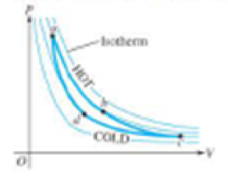
Figure 16.17
Problem 21.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
College Physics (10th Edition)
Additional Science Textbook Solutions
Conceptual Physical Science (6th Edition)
The Cosmic Perspective
The Cosmic Perspective (8th Edition)
Sears And Zemansky's University Physics With Modern Physics
The Cosmic Perspective Fundamentals (2nd Edition)
- An electric generating station is designed to have an electric output power of 1.40 MW using a turbine with two-thirds the efficiency of a Carnot engine. The exhaust energy is transferred by heat into a cooling tower at 110C. (a) Find the rate at which the station exhausts energy by heat as a function of the fuel combustion temperature Th. (b) If the firebox is modified to run hotter by using more advanced combustion technology, how does the amount of energy exhaust change? (c) Find the exhaust power for Th = 800C. (d) Find the value of Th for which the exhaust power would be only half as large as in part (c). (e) Find the value of Th for which the exhaust power would be one-fourth as large as in part (c).arrow_forward(a) What is the best coefficient of performance for a heat pump that has a hot reservoir temperature of 50.0C and a cold reservoir temperature of 20.0C ? (b) How much heat transfer occurs into the warm environment if 3.60107J of work (10.0kWh) is put into it? (c) If the cost of this work input is 10.0cent/kWh, haw does its cost compare with the direct heat transfer achieved by burning natural gas at a cost of 85.0 cents per therm. (A therm is a common unit of energy for natural gas and equals 1.055108J .)arrow_forwardA heat pump used for heating shown in Figure P18.25 is essentially an air conditioner installed backward. It extracts energy from colder air outside and deposits it in a warmer room. Suppose the ratio of the actual energy entering the room to the work done by the devices motor is 10.0% of the theoretical maximum ratio. Determine the energy entering the room per joule of work done by the motor given that the inside temperature is 20.0C and the outside temperature is 5.00C. Figure P18.25arrow_forward
- A heat engine operates between two temperatures such that the working substance of the engine absorbs 5000 J of heat from the high-temperature bath and discharges 3000 J to the low-temperature bath. The rest of the energy is converted into mechanical energy of the turbine. Find (a) the amount of work produced by the engine and (b) the efficiency of the engine.arrow_forwardIf a 35.0% -efficient Carnot heat engine (Fig. 21.2) is run in reverse so as to form a refrigerator (Fig. 21.4), what would be this refrigerators coefficient of performance? Figure P21.2 Schematic representation of a heat engine. Figure P21.4 Schematic representation of a heat pump.arrow_forwardSuppose an ideal (Carnot) heal pump could be constructed, (a) Using Equation 12.15, obtain an expression for the coefficient of performance for such a heat pump in terms of Th and Tc. (b) Would such a heal pump work better If the difference in the operating temperatures were greater or smaller? (c) Compute the coefficient of performance for such a heat pump if the cold reservoir is 50.0C and indoor temperature is 70.0C.arrow_forward
- A sample of a monatomic ideal gas is contained in a cylinder with a piston. Its state is represented by the dot in the PV diagram shown in Figure OQ18.9. Arrows A through E represent isobaric, isothermal, adiabatic, and isovolumetric processes that the sample can undergo. In each process except D, the volume changes by a factor of 2. All five processes are reversible. Rank the processes according to the change in entropy of the gas from the largest positive value to the largest-magnitude negative value. In your rankings, display any cases of equality. Figure OQ18.9arrow_forwardAn ideal gas is taken from an initial temperature Ti to a higher final temperature Tf along two different reversible paths. Path A is at constant pressure, and path B is at constant volume. What is the relation between the entropy changes of the gas for these paths? (a) SA SB (b) SA = SB (c) SA SBarrow_forward(a) How much heat transfer occurs from 20.0 kg of 90.0C water placed in contact with 20.0 kg of 10.0C water, producing a final temperature of 50.0C ? (b) How much work could a Carnot engine do with this heat transfer, assuming it operates between two reservoirs at constant temperatures of 90.0C and 10.0C ? (c) What increase in entropy is produced by mixing 20.0 kg of 90.0C water with 20.0 kg of 10.0C water? (d) Calculate the amount of work made unavailable by this mixing using a low temperature of 10.0C, and compare it with the work done by the Garnet engine. Explicitly show how you follow the steps in the Problem-Solving Strategies for Entropy. (e) Discuss how everyday processes make increasingly more energy unavailable to do work, as implied by this problem.arrow_forward
- Suppose you build a two-engine device with the exhaust energy output from one heat engine supplying the input energy for a second heat engine. We say that the two engines arc running in series. Let e1 and e2 represent the efficiencies of the two engines. (a) The overall efficiency of the two-engine device is defined as the total work output divided by the energy put into the first engine by heat. Show that the overall efficiency e is given by e=e1+e2e1e2 What If? For parts (b) through (e) that follow, assume the two engines are Carnot engines. Engine 1 operates between temperatures Th and Ti. The gas in engine 2 varies in temperature between Ti and Tc. In terms of the temperatures, (b) what is the efficiency of the combination engine? (c) Does an improvement in net efficiency result from the use of two engines instead of one? (d) What value of the intermediate temperature Ti results in equal work being done by each of the two engines in series? (e) What value of Ti results in each of the two engines in series having the same efficiency?arrow_forwardAn ideal gas with specific heat ratio confined to a cylinder is put through a closed cycle. Initially, the gas is at Pi, Vi, and Ti. First, its pressure is tripled under constant volume. It then expands adiabatically to its original pressure and finally is compressed isobarically to its original volume. (a) Draw a PV diagram of this cycle. (b) Determine the volume at the end of the adiabatic expansion. Find (c) the temperature of the gas at the start of the adiabatic expansion and (d) the temperature at the end of the cycle. (e) What was the net work done on the gas for this cycle?arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning





