
Concept explainers
(a)
Interpretation:
The species given in equations are to be labelled as acid or base.
Concept introduction:
According to Arrhenius, acid is a substance which produces hydrogen ion and Base is a substance which produces hydroxyl ions in an aqueous solution.
According to Bronsted-Lowry, acid is a substance which donate hydrogen ion and Base is a substance which accept hydrogen ion.
(a)
Answer to Problem 52A
In equation-I:
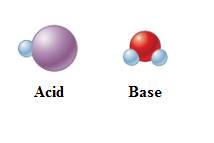
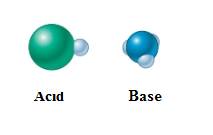
In equation-II:
Explanation of Solution
Equation-I:
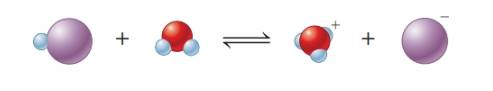
The given equation shows the first species gains negative charge. Therefore, it has lost

The given equation shows the first species gains positive charge. Therefore, it has gained
(b)
Interpretation:
The species given in equations are to be labelled as acid or base based on specific theory.
Concept introduction:
According to Arrhenius, acid is a substance which produces hydrogen ion and Base is a substance which produces hydroxyl ions in an aqueous solution.
According to Bronsted-Lowry, acid is a substance which donate hydrogen ion and Base is a substance which accept hydrogen ion.
(b)
Answer to Problem 52A
In equation-I:
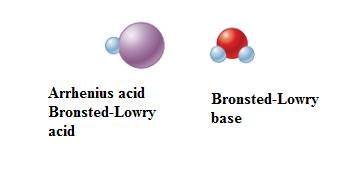
In equation-II:
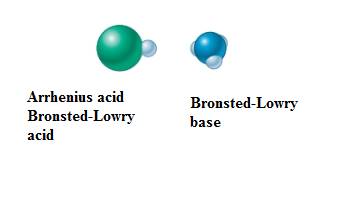
Explanation of Solution
Equation-I:
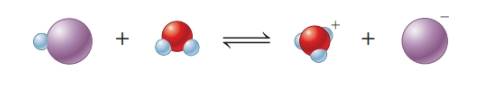
The given equation shows the first species gains negative charge. Therefore it has lost

The given equation shows the first species gains positive charge. Therefore, it has gained
Chapter 16 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





