
Organic Chemistry
7th Edition
ISBN: 9780321826596
Author: Bruice, Paula Yurkanis/
Publisher: Pearson College Div
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 73P
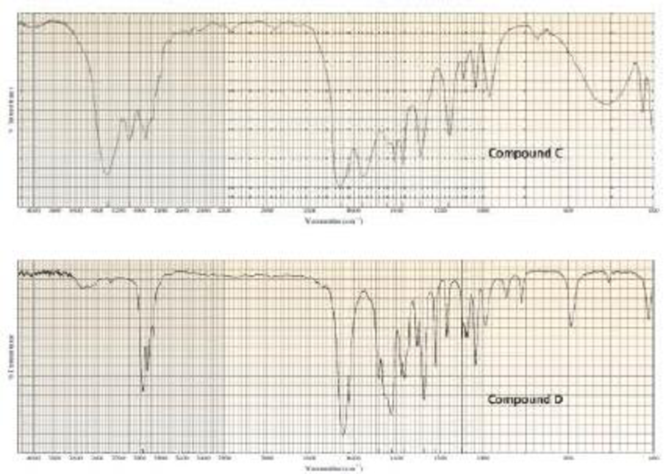
Two products, A and B, are obtained from the reaction of 1-bromobutane with NH3. Compound A reacts with acetyl chloride to form C, and compound B reacts with acetyl chloride to form D. The IR spectra of C and D are shown. Identify A, B, C, and D.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Benzene is one of the compounds used as octane enhancers in unleaded gasoline. It is manufactured by thecatalytic conversion of acetylene to benzene: 3C2 H2(g) ⇌ C6 H6(g). Which value of Kc would make this reactionmost useful commercially? Kc ≈ 0.01, Kc ≈ 1, or Kc ≈ 10. Explain your answer
The IR spectrum of compound A with a molecular formula of C5H12O is shown below. Compound A is oxidized to give compound B, a ketone with a molecular formula of C5H10O. When compound A is heated with H2SO4, compounds C and D are obtained. Considerably more D is obtained than C.Reaction of compound C with O3, followed by treatment with dimethyl sulfide, gives two products: formaldehyde and compound E, with a molecular formula of C4H8O. Reaction of compound D with O3, followed by treatment with dimethyl sulfide, gives two products: compound F, with a molecular formula of C3H6O, and compound G, with a molecular formula of C2H4O. What are the structures of compounds A through G?
Cyclohexene has the formula C6H1o and the structure shown in Figure 4-4. When cyclohexene is treated with acid and water it forms Compound A with the formula C6H12O. When Compound A is treated with an oxidizing agent, it forms Compound B with the formula C6H100.
IR spectra for Compounds A and B are shown in Figure 4-3. The best structure
for A is
The best for B is
Chapter 16 Solutions
Organic Chemistry
Ch. 16.1 - The aromas of many flowers and fruits are due to...Ch. 16.1 - Prob. 2PCh. 16.1 - Name the following:Ch. 16.1 - Prob. 4PCh. 16.2 - Prob. 5PCh. 16.2 - Which is longer, the carbon-oxygen single bond in...Ch. 16.2 - There are three carbon-oxygen bonds in methyl...Ch. 16.2 - Prob. 8PCh. 16.4 - Prob. 9PCh. 16.4 - Prob. 10P
Ch. 16.5 - a. What is the product of the reaction of acetyl...Ch. 16.6 - a. Which compound has the stretching vibration for...Ch. 16.6 - Using the pKa values listed in Table 15.1, predict...Ch. 16.6 - Is the following statement true or false? If the...Ch. 16.7 - What is the product of an acyl substitution...Ch. 16.8 - PROBLEM 16
Starting with acetyl chloride, what...Ch. 16.8 - Prob. 18PCh. 16.9 - We saw that it is necessary to use excess amine in...Ch. 16.9 - Prob. 20PCh. 16.9 - a. state three factors that cause the uncatalyzed...Ch. 16.10 - Prob. 23PCh. 16.10 - Using the mechanism for the acid-catalyzed...Ch. 16.10 - Prob. 25PCh. 16.10 - Prob. 27PCh. 16.10 - Write the mechanism for the acid-catalyzed...Ch. 16.10 - Write the mechanism for the acid-catalyzed...Ch. 16.11 - Prob. 30PCh. 16.12 - Prob. 31PCh. 16.12 - Prob. 32PCh. 16.13 - Prob. 34PCh. 16.13 - Which has a higher melting point, glyceryl...Ch. 16.13 - Draw the structure of an optically inactive fat...Ch. 16.13 - Draw the structure of an optically active fat...Ch. 16.14 - Show how each of the following esters could he...Ch. 16.14 - Prob. 39PCh. 16.15 - Prob. 40PCh. 16.15 - Which of the following reactions leads to the...Ch. 16.16 - Prob. 42PCh. 16.16 - Prob. 43PCh. 16.18 - Prob. 44PCh. 16.18 - Prob. 45PCh. 16.19 - Prob. 46PCh. 16.19 - Which alkyl halides from the carboxylic acids...Ch. 16.20 - Prob. 49PCh. 16.20 - Prob. 50PCh. 16.20 - Prob. 51PCh. 16.21 - Prob. 52PCh. 16.21 - Prob. 53PCh. 16.22 - How could you synthesize the following compounds...Ch. 16 - Prob. 55PCh. 16 - Name the following:Ch. 16 - Prob. 57PCh. 16 - What compound are obtained from the fallowing...Ch. 16 - a. Rank the following esters in order of...Ch. 16 - Because bromocyclohexane is a secondary alkyl...Ch. 16 - a. Which compound would you expect to have a...Ch. 16 - How could you use 1H NMR spectroscopy to...Ch. 16 - Prob. 63PCh. 16 - a. When a carboxylic acid is dissolved in...Ch. 16 - Rank the following compounds in order of...Ch. 16 - Prob. 66PCh. 16 - Prob. 67PCh. 16 - Prob. 68PCh. 16 - A compound with molecular formula C5H10O2 gives...Ch. 16 - Prob. 70PCh. 16 - Prob. 71PCh. 16 - Prob. 72PCh. 16 - Two products, A and B, are obtained from the...Ch. 16 - Prob. 74PCh. 16 - Prob. 75PCh. 16 - Prob. 76PCh. 16 - Prob. 77PCh. 16 - When treated with an equivalent of methanol,...Ch. 16 - a. Identify the two products obtained from the...Ch. 16 - Prob. 80PCh. 16 - Identity the major and minor products of the...Ch. 16 - Prob. 82PCh. 16 - When a compound with molecular formula C11H14O2...Ch. 16 - Prob. 84PCh. 16 - Prob. 85PCh. 16 - The 1H NMR spectra for two esters with molecular...Ch. 16 - Show how the following compounds could be prepared...Ch. 16 - Prob. 88PCh. 16 - Prob. 89PCh. 16 - Prob. 90PCh. 16 - The intermediate shown here is formed during the...Ch. 16 - Prob. 92PCh. 16 - Propose a mechanism that accounts for the...Ch. 16 - Catalytic antibodies catalyze a reaction by...Ch. 16 - Prob. 95P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ketones undergo a reduction when treated with sodium borohydride, NaBH4. What is the structure of the compound produced by reaction of 2-butanone with NaBH4 if it has an IR absorption at 3400 cm-1 and M+=74 in the mass spectrum?arrow_forwardThe 1H and 13C NMR spectra of compound A, C8H9Br are shown below. Answer the following questions. 1(a) Degree of the unsaturation of this compound is = , 1(b) The derived unsaturation number indicates that compound has ............= 1(c) Two peaks in between 6.5 - 8.0 δ indicate that compound is= 1(d) According to the splitting pattern of the peak at 1.20 δ and 2.58 δ indicates that compound has a .................. group= 1(e) According to the 1H NMR spectrum the number of nonequivalent aromatic proton sets in the compound = 1(f) According to the 13C NMR, the number of nonequivalent carbons in the compound is = 1(g) According to your answer in Q 1(f) the compound has a plane of symmetry Yes or NO = 1(h) The IUAC name for this unknown compound isNOT TOO SURE ABOUT MY ANSWERS, PLEASE CORRECT ME IF I'M WRONGarrow_forwardCompounds B and C are isomers with molecular formula C5H9BrO2. The 1H NMR spectrum of compounds B and C are shown below. The IR spectrum corresponding to compound B showed strong absorption bands at 1739, 1225, and 1158 cm-1, while the spectrum corresponding to compound C have strong bands at 1735, 1237, and 1182 cm-1. 1.Based on the information provided, determine the structure of compounds B and C. 2.Assign all peaks in 1H NMR spectrum of compounds B and C.arrow_forward
- The p-toluenesulfonate derived from (R)-2-octanol and p-toluenesulfonyl chloride was allowed to react with sodium benzenethiolate (C6H5SNa). Give the structure, including stereochemistry and the appropriate R or S descriptor, of the product.arrow_forwardTreatment of alcohol A (molecular formula C5H12O) with CrO3, H2SO4, and H2O affords B with molecular formula C5H10O, which gives an IR absorption at 1718 cm−1. The 1H NMR spectrum of B contains the following signals: 1.10 (doublet, 6 H), 2.14 (singlet, 3 H), and 2.58 (septet, 1 H) ppm. What are the structures of A and B?arrow_forwardA chiral ether of molecular formula C5H10O reacts with hot HI to give a product of molecular formula C5H10I2. Treatment of this compound with hot potassium tert-butoxide produces 1,3-pentadiene. determine the structure of the original ether?arrow_forward
- Treatment of compound C (molecular formula C9H12O) with PCC affords D (molecular formula C9H10O). Use the 1H NMR and IR spectra of D to determine the structures of both C and D.arrow_forwardA. In the synthesis of 1-bromobutane, what is the inorganic by-product left in the reaction flask following the distillation? Why was the bromoalkane the bottom layer in the separatory funnel? B. Predict the product when 1-methylcyclohexanol reacts with H2SO4 and KBr. Show the mechanism.arrow_forwardAcid-catalyzed hydrolysis of HOCH2CH2C(CH3)2CN forms compound A (C6H10O2). A shows a strong peak in its IR spectrum at 1770 cm-1 and the following signals in its 1H NMR spectrum: 1.27 (singlet, 6 H), 2.12 (triplet, 2 H), and 4.26 (triplet, 2 H) ppm. Draw the structure for A and give a stepwise mechanism that accounts for its formation.arrow_forward
- Treatment of compound E (molecular formula C4H8O2) with excessCH3CH2MgBr yields compound F (molecular formula C6H14O) afterprotonation with H2O. E shows a strong absorption in its IR spectrum at1743 cm−1. F shows a strong IR absorption at 3600−3200 cm−1. The 1HNMR spectral data of E and F are given. What are the structures of E andF?Compound E signals at 1.2 (triplet, 3 H), 2.0 (singlet, 3 H), and 4.1 (quartet, 2 H) ppm Compound F signals at 0.9 (triplet, 6 H), 1.1 (singlet, 3 H), 1.5 (quartet, 4H), and 1.55 (singlet, 1 H) ppmarrow_forwardA chiral ether of molecular formula C5H10O reacts with hot HI to give a product of molecular formula C5H10I2. Treatment of this compound with hot potassium tert-butoxide produces 1,3-pentadiene. What is the structure of the original ether?arrow_forward19. A compound with molecular formula C6H12O2 exhibits two singlets in its 1H NMR spectrum, at d 1.4 (9H) and d 2.0 (3H). Its IR spectrum shows a strong absorption band near 1740 cm-1. What is the structure for this compound? Show the correlation of the spectra with the molecular structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY