
Concept explainers
(a)
Interpretation: Considering the base hydrolysis of given ester answer the follwoing questions.
(a) The product which contains labelled oxygen atom
Concept introduction:
The hydrolysis reaction of an ester can be catalyzed in presence of a base that is hydroxide ion. The
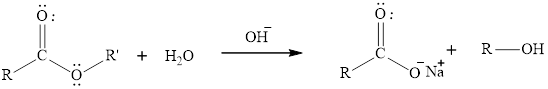
Here, the ester molecule contains a labelled oxygen atom
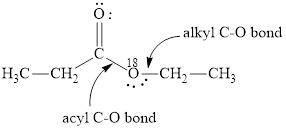
(b)
Interpretation:
Considering the base hydrolysis of given ester answer the follwoing questions.
(b) The product which contains labelled oxygen atom
Concept introduction:
The hydrolysis reaction of an ester can be catalyzed in presence of a base that is hydroxide ion. The rate of reaction increase due to the presence of hydroxide ion which can act as better nucleophile than water molecule. Thus substitution of alkoxide group takes place and carboxylic acid formation occur. The general reaction of base hydrolysis of en ester is written as,
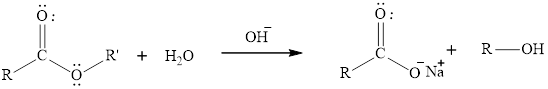
Here, the ester molecule contains a labelled oxygen atom

Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Organic Chemistry
- When methanitrobenzoic acid is reacted with bromomethane in the presence of Lewis acids, the product is: A. III No.B. V numbered structureC. Structure ID. Building IIE. Building IVarrow_forwardHow is an amide converted to an anhydride? Water with strong acid → PCl5 → Carboxylic Acid PCl5 → Water with strong acid → Carboxylic Acid Water with strong acid → Carboxylic Acid → PCl5 PCl5 → Carboxylic Acid → Water with strong acid What predominant electronic effect makes the amide a bad leaving group? + I - I + M - Marrow_forwardThe reaction of a nitrile with an alcohol in the presence of a strong acid forms an N-substituted amide. This reaction, known as the Ritter reaction, does not work with primary alcohols. a. Propose a mechanism for the Ritter reaction. b. Why does the Ritter reaction not work with primary alcohols? c. How does the Ritter reaction differ from the acid-catalyzed hydrolysis of a nitrile to form an amide?arrow_forward
- Four of the seven steps in the mechanism for this process are shown in the conversion of acetal A to hemiacetal E. a.) Add curved arrows for each step b.) Draw another resonance structure for C.c.) Identify the nucleophile and electrophile in Step [3].d.) Which steps are Brønsted–Lowry acid–base reactions?arrow_forwardDraw the mechanisms for the reactions between the given carboxylic acid and LDA in THF at -78 deg C.arrow_forward1(a) Draw the enolate cycle for keto-enol tautomerism in ester. 2) Complete the claisen condensation reactions for the following: a) Ethlypropanoate and 2-Methlyethylbutanoate b) 2-Cloromethylbutanoate and propylbenzoatearrow_forward
- What does the reaction of 4-Chloropentan-2-amine reacted with excess CH3l, Ag2O produce? O Hofmann product only O Zaitsev product only O Both Hofmann and Zaitsev products Equal distributionarrow_forwardWhat is the best choice of reagents to achieve the following reaction? A. SOCl2 (1/2 equivalent) B. KOH (pellets) C. K2SO4 D. PhCO2Naarrow_forwardThe reaction of a nitrile with an alcohol in the presence of a strong acid forms an N-substituted amide. This reaction, known as the Ritter reaction, doesnot work with primary alcohols. a. Why does the Ritter reaction not work with primary alcohols? b. Provide an explanation for why an amide is less susceptible to nucleophilic attack than its corresponding ester.arrow_forward
- Draw the products formed when benzoyl chloride (C6H5COCl) is treated with each nucleophile: (a) H2O, pyridine; (b) CH3COO-; (c) NH3 (excess); (d) (CH3)2NH (excess).arrow_forwardDraw the product of the following compounds with the given image. 1. H2O, H+ 2. m-CPBA 3. cold dilute KMnO4arrow_forward9. Product of the reaction of nitrobenzene with sulfuric acid in the presence of oleum at 60 °C:a) o-nitro benzenesulfonic acidb) m-nitro benzenesulfonic acidc) p-nitro benzenesulfonic acidd) None of the above 10. Due to their ability to move through ducts or pipes, they are generally called fluids:a) Solidsb) Solid mixturesc) Gasesd) Liquids and gasesarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

