
a) Bromobenzene
Interpretation:
The major product(s) formed when bromobenzene is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, excep halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituent groups are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituent groups are less reactive than benzene.
To give:
The major products formed when bromobenzene is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major products formed when bromobenzene is nitrated are o-bromonitrobenzene and p-bromonitrobenzene.
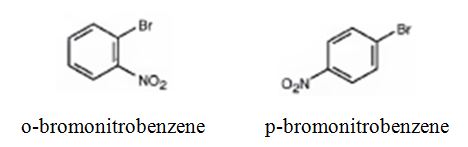
Bromobenzene will react slower than benzene.
Explanation of Solution
Bromine is an o- and p-directing group. It also has considerable electron withdrawing inductive effect which deactivates the ring. Hence bromobenzene is less reactive than benzene.
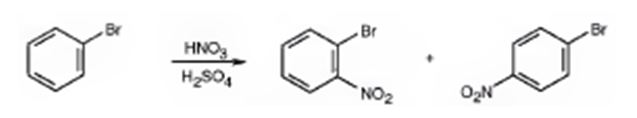
The major products formed when bromobenzene is nitrated are o-bromonitrobenzene and p-bromonitrobenzene.
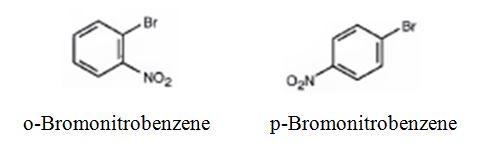
Bromobenzene will react slower than benzene.
b) Benzonitrile
Interpretation:
The major product(s) formed when benzonitrile is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when benzonitrile is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major product formed when benzonitrile is nitrated is m-nitrobenzonitrile.
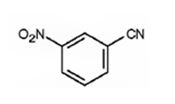
Benzonitrile will react slower than benzene.
Explanation of Solution
The cyanide group is strongly electron withdrawing in nature. Hence it is a meta director. The attraction of electrons away from the ring reduces the electron density in the ring. Thus benzonitrile reacts slower than benzene.
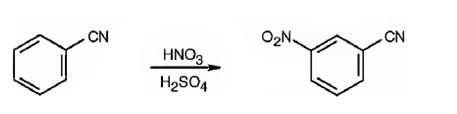
The major product formed when benzonitrile is nitrated is m-nitrobenzonitrile.

Benzonitrile will react slower than benzene.
c) Benzoic acid
Interpretation:
The major product(s) formed when benzoic acid is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when benzoic acid is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major product formed when benzoic acid is nitrated is m-nitrobenzoic acid.
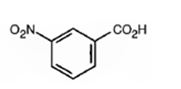
Benzoic acid will react slower than benzene.
Explanation of Solution
The C=O in carboxyl group is strongly electron withdrawing in nature. Hence it is a meta director. The attraction of electrons away from the ring reduces the electron density in the ring. Thus benzoic acid reacts slower than benzene.
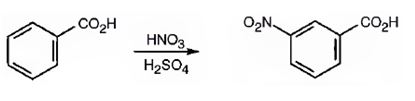
The major product formed when benzoic acid is nitrated is m-nitrobenzoic acid.

Benzoic acid will react slower than benzene.
d) Nitrobenzene
Interpretation:
The major product(s) formed when nitrobenzene is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when nitrobenzene is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major product formed when nitrobenzene is nitrated is m-dinitrobenzene.
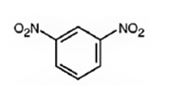
Nitrobenzene will react slower than benzene.
Explanation of Solution
The nitro group is strongly electron withdrawing in nature. Hence it is a meta director. The attraction of electrons away from the ring reduces the electron density in the ring. Thus nitrobenzene reacts slower than benzene.
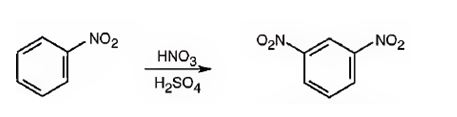
The major product formed when nitrobenzene is nitrated is m-dinitrobenzene.

Nitrobenzene will react slower than benzene.
e) Benzenesulfonic acid
Interpretation:
The major product(s) formed when benzenesulfonic acid is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when benzenesulfonic acid is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major product formed when benzenesulfonic acid is nitrated is m-nitro benzenesulfonic acid.
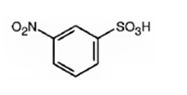
Benzenesulfonic acid will react slower than benzene.
Explanation of Solution
The sulfonic acid group is strongly electron withdrawing in nature. Hence it is a meta director. The attraction of electrons away from the ring reduces the electron density in the ring. Thus benzenesulfonic acid reacts slower than benzene.
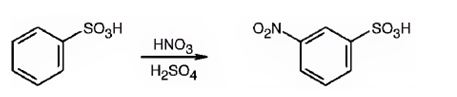
The major product formed when benzenesulfonic acid is nitrated is m-nitro benzenesulfonic acid.

Benzenesulfonic acid will react slower than benzene.
f) Methoxybenzene
Interpretation:
The major product(s) formed when methoxybenzene is nitrated is to be given. Whether it will react faster or slower than benzene is also to be stated.
Concept introduction:
Monosubstituted benzenes can be nitrated using a mixture of Conc. HNO3 and H2SO4. Electron releasing substituent groups, except halogens, activate the ring and direct the incoming electrophile to the o- and p-positions. Compounds with these substituents are more reactive than benzene. Halogens are o- and p-directors but they deactivate the ring. Hence halobenzenes are less reactive than benzene. Electron withdrawing substituent groups deactivate the ring and direct the incoming electrophile to the m-position. Compounds with these substituents are less reactive than benzene.
To give:
The major products formed when methoxybenzene is nitrated and to state whether it will react faster or slower than benzene.
Answer to Problem 47AP
The major products formed when methoxybenzene is nitrated are o-nitromethoxybenene and p-nitromethoxybenene.
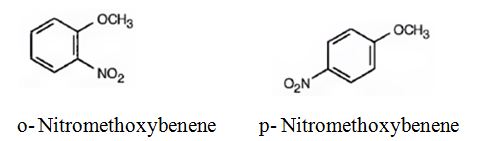
Methoxybenzene will react faster than benzene.
Explanation of Solution
The methoxy group is electron releasing in nature. Hence it is an o- and p-director. The attraction of electrons towards the ring increases the electron density in the ring. Thus methoxybenzene reacts faster than benzene.
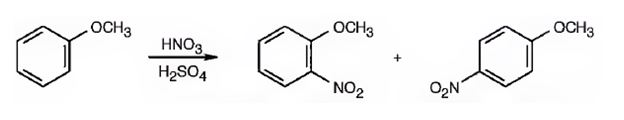
The major products formed when methoxybenzene is nitrated are o-nitromethoxybenene and p-nitromethoxybenene.
Methoxybenzene will react faster than benzene.
Want to see more full solutions like this?
Chapter 16 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- Provide the missing reagents to complete the reactions below, if there is more than one step required, be sure to indicate appropriately.arrow_forwardArrange the compounds in order of INCREASING reactivity towards bromination. I. Toluene, Nitrobenzene, Iodobenzene, Acetanilide II. Phenol, Acetophenone, Anisole, Chlorobenzene III. Aniline, Benzaldehyde, Benzonitrile, Benzenesulfonic acidarrow_forwardWhat products would you would expect to obtain when the following compounds react with ozone and then with dimethylsulfide?arrow_forward
- A common illicit synthesis of methamphetamine involves an interesting variation of the Birch reduction. A solution of ephedrine in alcohol is added to liquid ammonia, followed by several pieces of lithium metal. The Birch reduction usually reduces the aromatic ring, but in this case it eliminates the hydroxy group of ephedrine to give methamphetamine. Propose a mechanism, similar to that for the Birch reduction, to explain this unusual course of the reaction.arrow_forwardIn an aqueous solution containing sodium bicarbonate, aniline reacts quickly with bromine to give 2,4,6-tribromoaniline. Nitration of aniline requires very strong conditions, however, and the yields (mostly m-nitroaniline) are poor.Explain why nitration of aniline is so sluggish and why it gives mostly metasubstitution.arrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)p-Chlorobenzoic acidarrow_forward
- Suggest ways of synthesizing the following compounds, how would youmake the disconnections and what are the synthons?arrow_forwardSuggest a suitable mechanism for this multi-step reaction, and give products A and Barrow_forward(19, 8, 1) Specify a synthetic scheme that would produce the compound shown above in the fewest steps possible. Use one of the starting materials shown together with any of the available reagents. Give the number of the starting material followed by the letters of the reagents in the order of their use, for example: 3be.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

