
a)

Interpretation:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 2-bromo-4-nitrotoluene can be synthesized is to be stated.
Concept introduction:
In
Electrophilic substitution of di and trisubstituted benzenes follows three simple rules. (i) If the directing influence of both the substituents reinforce each other, a single product results. (ii) If the directing influences of both the substituent groups oppose each other, the most powerful activating group among them has the dominant influence but usually a mixture of products results. (iii) In meta disubstituted compounds, further substitution in between the groups occurs only rarely, due to steric reasons.
To state:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 2-bromo-4-nitrotoluene can be synthesized.
b)

Interpretation:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 1,3,5-trinitrobenzene can be synthesized is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions the nitro group is a meta directing group. Nitration of benzene gives nitrobenzene. Further nitration of nitrobenzene will introduce nitro groups into the two meta positions to yield the trinitro compound.
To state:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 1,3,5-trinitrobenzene can be synthesized.
c)
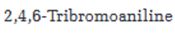
Interpretation:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 2,4,6-tribromoaniline can be synthesized is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, the amino group is an ortho and para directing group. Nitration of benzene gives nitrobenzene. Nitrobenzene can be reduced to aminobenzene (aniline). Aniline upon bromoination will yield 2,4,6-tribromoaniline.
To state:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 2,4,6-tribromoaniline can be synthesized.
d)
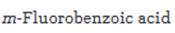
Interpretation:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how m-fluorobenzoic acid can be synthesized is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions the carboxylic acid group is a meta directing group. Hence to prepare m- fluorobenzoic acid, benzene should be first converted into benzoicacid. Direct fluorination of aromatic rings is not possible as fluorine is too reactive and it is not possible to stop the reaction at the monofluorinated stage. Fluorination is normally carried out using F-TEDA-BF4 which has a fluorine atom bonded to positively charged nitrogen.
To state:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how m-fluorobenzoic acid can be synthesized.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Bundle: Organic Chemistry, Loose-leaf Version, 9th + LMS Integrated for OWLv2, 4 terms (24 months) Printed Access Card
- Three isomeric pentanols with unbranched carbon chains exist. Which of these isomers, upon dehydration at 180C, yields only 1-pentene as a product?arrow_forward3. Cyclopropenones are described as having aromatic character. How would you account for this, given that the ring contains three π-electrons?arrow_forwardA compound A, C7H12, was found to be optically active. On catalytic reduction over platinum catalyst, 2 equivalents of hydrogen were absorbed, yielding compound B, C7H16. On ozonolysis of A, two fragments were obtained. One fragment was identified as acetic acid. The other fragment, compound C, was an optically active carboxylic acid, C5H10O2. Write the reactions and draw structures for A, B and C.arrow_forward
- Compound A is an alcohol that undergoes oxidation to produce compound B.Compound B is a ketone that gives positive triiodomethane reaction. Compound B isthen reacted with phenyl magnesium bromide, C6H5MgBr in the presence of aqueousacid to form compound C. Compound C has the molecular formula of C9H12O. Deducethe structure for compound A, B and C. PLEASE PROVIDE CLEAR DRAWINGS AND EXPLANATIONSarrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)2,4,6-Trinitrotoluene (TNT)arrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)m-Nitrobenzenesulfonic acidarrow_forward
- Grignard reagent is a versatile tool in synthetic organic chemistry. Using bromocyclopentane as a starting material, show how a Grignard reagent, X, is synthesized. Reaction of X with water produces compound Y while treatment in carbon dioxide followed by hydrolysis forms compound Z. 3-methyl-2butanone reacts with X and hydrolyses to yield compound AA. Draw the structural formulae of compounds Y, Z and AA and write the chemical equations respectively.arrow_forwardCadaverine (1,5-diaminopentane) and putrescine (1,4-diaminobutane) are two compounds that are formed by bacterialaction and are responsible for the odor of rotting flesh. Drawtheir structures. Suggest a series of reactions to synthesize pu-trescine from 1,2-dibromoethane and any inorganic reagentsarrow_forwardCompound A (C11H23Br) is a secondary alkyl halide. On being heated with a solution of sodium ethoxide in ethanol, compound A yielded a mixture of two alkenes B and C, each having molecular formula C11H22. Catalytic hydrogenation of the major isomer B or the minor isomer C gave only 3,5-diethylheptane. Draw structures for compounds A, B, and C consistent with these observations.arrow_forward
- Wolff-Kishner reduction of compound W gave compound A. Treatment of A with m-chloroperbenzoic acid gave B which on reduction with LiAH4 gave C. Oxidation of compound C with chromic acid gave D (C9H14O). Suggest the structures for A, B, C, and D.arrow_forwardTwo moles of organic compound ‘A’ on treatment with a strong base gives two compounds ‘B’ and ‘C’. Compound ‘B’ on dehydrogenation with Cu gives ‘A’ while acidification of ‘C’ yields carboxylic acid ‘D’ with molecular formula of CH2O2. Identify the compounds A, B, C and D and write all chemical reactions involved.arrow_forwardCompound A (C6H12O2) reacts with water, acid, and heatto yield compound B (C5H10O2) and compound C (CH4O).Compound B is acidic. Deduce possible structures of compounds A, B, and Carrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


