
(a)
Interpretation:
The product has to be written for the reaction involving excess
Concept Introduction:
Esters can be simply represented as,
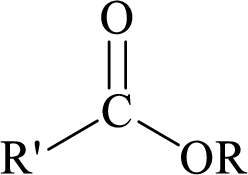
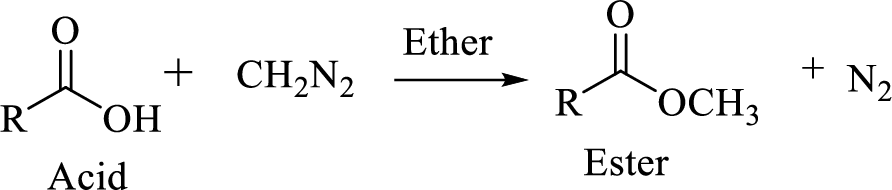
Carboxylic acids can be converted to their methyl esters by adding an ether/alcoholic solution of diazomethane.
(b)
Interpretation:
The product has to be written for the reaction involving excess
Concept Introduction:
Esters can be simply represented as,
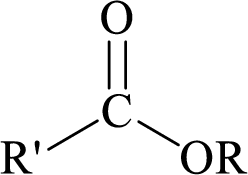
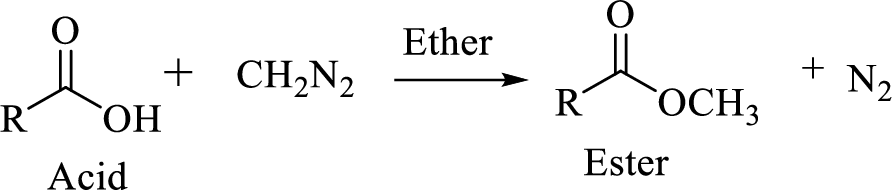
Carboxylic acids can be converted to their methyl esters by adding an ether/alcoholic solution of diazomethane.
(c)
Interpretation:
The product has to be written for the reaction involving excess
Concept Introduction:
Esters can be simply represented as,
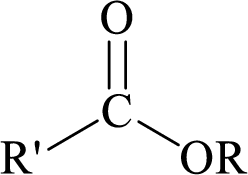
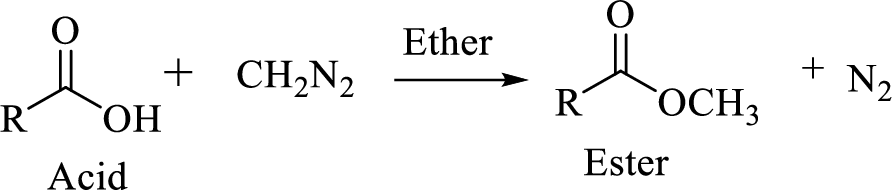
Carboxylic acids can be converted to their methyl esters by adding an ether/alcoholic solution of diazomethane.
Trending nowThis is a popular solution!

Chapter 17 Solutions
Organic Chemistry
- When the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardA compound with formula C7H12O is treated with sodium borohydride in methanol to yield 2,2-dimethylcylopentanol. Write a reaction scheme showing the structures of the reactant, the reagents, and the product. Will the product be optically active? Explain.arrow_forwardThe acid-catalyzed dehydration of 2,3-dimethyl-3-pentanol yields three alkene products. What are the names of the three alkenes? Which of the three alkenes is the major product?arrow_forward
- What happens when(i) Chlorobenzene is treated with Cl2/FeCl3,(ii) Ethyl chloride is treated with AgNO2,(iii) 2-bromopentane is treated with alcoholic KOH?Write the chemical equations in support of your answer.arrow_forwardWhen trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanone i. Write the equation for the reaction between trans-2-chloro-1-cyclohexanol and the base to yield the cyclohexene oxide. ii. Why doesn’t the cis isomer yield the oxide?. iii. Write the mechanism for each of the two reactions.arrow_forwardDraw the structures for and give the names for each of the isomeric carbonyl compounds corresponding to C4H8O and suggest suitable reactions for distinguishing between themarrow_forward
- When trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanone. 1. Write the equation for the reaction between trans-2-chloro-1-cyclohexanol and the base to yield the cyclohexene oxide. 2. Why doesn't the cis isomer yield the oxide? 3. Write the mechanism for each of the two reactionsarrow_forwardDescribe a sequence of reactions by which cis-2-pentene could be prepared from acetylene.arrow_forwardThe ester with the formula C8H16O2 gives an alcohol and an acid when hydrolyzed. When the alcohol is isolated and oxidized, it forms a ketone. Which of these formulas cannot be the ester?arrow_forward
- Provide the products, intermediates, and/or reagents for the following reactions:arrow_forwardWhen trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanone A.Write the equation for the reaction between trans-2-chloro-1-cyclohexanol and the base to yield the cyclohexene oxide B.Why doesn’t the cis isomer yield the oxide? C.Write the mechanism for each of the two reactions.arrow_forward2-chloro-1-phenylpropane is reacted with ammonia, producing amphetamine, a widely misused stimulant. Write the chemical equation for the synthesis of amphetamine as described showing the structure of the compounds.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
