
Concept explainers
(a)
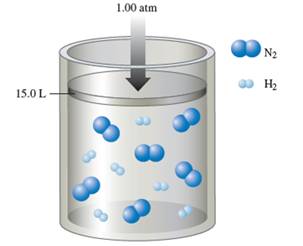
Interpretation: The partial pressure of ammonia in the container needs to be determined, when the reaction is complete at constant 1.00 atm and at constant volume.
Concept Introduction: The ideal gas equation is the equation which gives the relations between P,V, n and T of gases. It can be written as:
Usually gases do not follow ideal behavior under all conditions of pressure, temperature and volumes. The relation of P, V, n and T of real gases can be given by Vander Waals equation:
Hence
(a)
Answer to Problem 126CP
Explanation of Solution
Number of
Number of
The balance chemical equation for the formation of ammonia:
Hence 1 molecule of
Hence number of
Thus remaining
Number of ammonia molecule formed = 2 x 2 = 4 molecules.
Total molecules at completion of reaction = 4 +4 = 16 molecules
Calculate mole fraction of ammonia:
Calculate partial pressure of
(b)
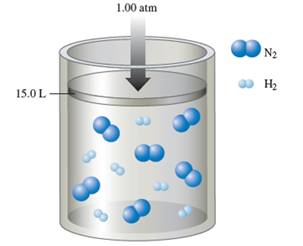
Interpretation: Interpret the mole fraction of ammonia in the container when the reaction is complete at constant 1.00 atm and at constant volume.
Concept Introduction: The ideal gas equation is the equation which gives the relations between P,V, n and T of gases. It can be written as:
Usually gases do not follow ideal behavior under all conditions of pressure, temperature and volumes. The relation of P, V, n and T of real gases can be given by Vander Waals equation:
Hence
(b)
Answer to Problem 126CP
Explanation of Solution
Number of
Number of
The balance chemical equation for the formation of ammonia:
Hence 1 molecule of
Hence number of
Thus remaining
Number of ammonia molecule formed = 2 x 2 = 4 molecules.
Total molecules at completion of reaction = 4 +4 = 16 molecules
Calculate mole fraction of ammonia:
(c)
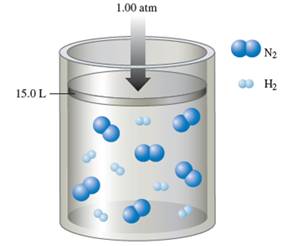
Interpretation:Interpret the volume of the container when the reaction complete if the initial pressure is 1.0 atm and volume is 15.0 L. The partial pressure of ammonia is 0.5 atm when reaction is complete.
Concept Introduction: The ideal gas equation is the equation which gives the relations between P,V, n and T of gases. It can be written as:
Usually gases do not follow ideal behavior under all conditions of pressure, temperature and volumes. The relation of P, V, n and T of real gases can be given by Vander Waals equation:
Hence
(c)
Answer to Problem 126CP
Volume = 10.0 L
Explanation of Solution
Initial volume is 15 L. Now, total initial moles are 2n. The final number of moles can be calculated by taking sum of moles of ammonia and moles of nitrogen.
At constant temperature, pressure can be calculated for an ideal gas as follows:
Putting the values,
Thus, the final volume is 10 L.
Want to see more full solutions like this?
Chapter 18 Solutions
Chemical Principles
- What possible uses exist for the natural gas liquids that are removed from natural gas during its processing?arrow_forwardWhat volume (ml) of hydrogen gas, saturated with water vapor, will be obtained from a 0.2218g sample alloy which is 27.77% Al (Atmospheric Pressure=762mmHg, ambient room temperature=21 degrees C, water vapor pressure=18.8mmHg).arrow_forwardAmmonia (NH3) is a weak base. Melting point of ammonia is -78 Celsius degrees and boiling point is -33 Celsius degrees. Therefore, pure ammonia appears in gaseous form in room temperature. a) Preparation of ammonia Gaseous ammonia (NH3) can be prepared by a reaction where Hydrogen gas (H2) and Nitrogen gas (N2) reacts. Write a reaction equation to this reaction. Balance the reaction equation if needed. b) Behavior of gases Explain the diffusion of gases. c) Change of temperature The closed container filled with gaseous ammonia is moved from room temperature into a freezer. The temperature on freezer is -35 Celsius degrees. Explain shortly on your own words, what kind of changes does the cooling cause and why.arrow_forward
- You have two glass bottles, one containing oxygen and onefilled with nitrogen. How could you determine which oneis which?arrow_forwardair pollution in the mexico city metropolitan area is among the worst in the world. the concentration of ozone in Mexico city has been measured at 441ppb (0.441ppm) mexico city sits at an altitude of 7400 feet, which measure its atmospheric pressure is 0.67atm calculate the partial pressure of ozone at 441ppb if the atmospheric pressure is 0.67arrow_forwardThe atmosphere on early Earth (4.5 to 3.8 billion years ago) __________. was formed by lightning energy and UV radiation allowed for the chemical processes that led to early life created unfavorable conditions for forming chemical bonds consisted of a combination of gases, including oxygenarrow_forward
- Cadmium selenide (CdSe) is used as a semiconductor quantum dot and is an example ofnanotechnology that is being developed for use as a theranostic.b) What physical chemical properties enable it to be developed as a theranostic?arrow_forwardOxygen gas formed by the decomposition of potassium chlorate was collected on the water. The volume of oxygen gas collected at 24 ºC and 762 mm-Hg pressure is 128 mL. Calculate the mass of potassium chlorate used in the reaction in grams. The pressure of the water vapor at 24 ºC is 22.4 mm-Hg. KClO3 (s) → KCl (s) + O2 (g)arrow_forwardConsider the reaction CaO (s) + CO2 (g) → CaCO3 (g) What mass of CO2 could be absorbed by 1.25 g of CaO? What volume would this CO2 occupy at STP?arrow_forward
- calculate the pressure (in atmospheres) at 11,000 meters. assume that the pressure at sea level is 1 ATM and that pressure increases at the rate of 1 ATM per every 10 metersarrow_forward(a) From the phase diagram for carbon dioxide, determine the state of co2 at 20 atm and 273 K. (b) Explain why co2 can undergo sublimation under normal condition.arrow_forwardSolid KClO3 decomposes, when heated.a. Write the balanced decomposition reaction for solid KClO3: b. How many liters of oxygen gas at 153°C and 0.820 atm can be produced by the decomposition of 2.560 g of solid KClO3?arrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





