
Concept explainers
(a)
Interpretation: The hybridization of nitrogen atom in
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The shape or geometry of molecule can be predicted with the help of hybridization and VSEPR theory. It can be checked with the below formula:
(a)
Answer to Problem 117AE
Explanation of Solution
To draw the Lewis structure of
ion:
Number of valence electrons in N = 5
Number of valence electrons in O = 6
Total number of valence electrons in
Hence the Lewis structure of
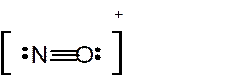
The hybridization can be checked with the help of Lewis structure and VSEPR theory which provide the geometry of molecules with lone pairs.
(b)
Interpretation: The hybridization of nitrogen atom in
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The shape or geometry of molecule can be predicted with the help of hybridization and VSEPR theory. It can be checked with the below formula:
(b)
Answer to Problem 117AE
Explanation of Solution
To draw the Lewis structure of
molecule :
Number of valence electrons in N = 5
Number of valence electrons in O = 6
Total number of valence electrons in
Hence the Lewis structure of
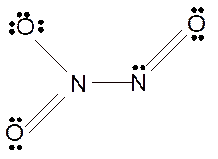
The hybridization can be checked with the help of Lewis structure and VSEPR theory which provide the geometry of molecules with lone pairs.
(c)
Interpretation: The hybridization of nitrogen atom in
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The shape or geometry of molecule can be predicted with the help of hybridization and VSEPR theory. It can be checked with the below formula:
(c)
Answer to Problem 117AE
Explanation of Solution
To draw the Lewis structure of
Number of valence electrons in N = 5
Number of valence electrons in O = 6
Total number of valence electrons in
Hence the Lewis structure of
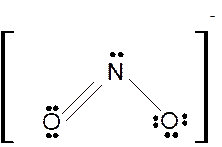
The hybridization can be checked with the help of Lewis structure and VSEPR theory which provide the geometry of molecules with lone pairs.
(d)
Interpretation: The hybridization of nitrogen atom in
Concept Introduction:
Lewis dot structure is the representation which shows the bonding between atoms present in a molecule. It shows lone pairs and bond pairs that existing on each bonded atom. Lewis dot structure is also known as Lewis dot formula or electron dot structure.
The shape or geometry of molecule can be predicted with the help of hybridization and VSEPR theory. It can be checked with the below formula:
(d)
Answer to Problem 117AE
Explanation of Solution
To draw the Lewis structure of
Number of valence electrons in N = 5
Total number of valence electrons in
Hence the Lewis structure of

The hybridization can be checked with the help of Lewis structure and VSEPR theory which provide the geometry of molecules with lone pairs.
Want to see more full solutions like this?
Chapter 18 Solutions
Chemical Principles
- What is the hybridization of iodine in IF3 and IF5?arrow_forwardHow can the paramagnetism of O2 be explained using the molecular orbital model?arrow_forwardDescribe the hybridization of boron and the molecular structure about the boron in each of the following: (a) H3BPH3. (b) BF4-. (c) BBr3. (d) B(CH3)3. (e) B(OH)3arrow_forward
- Why does the molecular orbital model do a better job in explaining the bonding in NO and NO than the hybrid orbital model?arrow_forwardPlatinum hexafluoride is an extremely strong oxidizing agent. It can even oxidize oxygen, its reaction with O2 giving O2+PtFt6. Sketch the molecular orbital energy level diagram for the O2+ ion. How many net and bonds does the ion have? What is the oxygen-oxygen bond order? How has the bond order changed on taking away electrons from O2 to obtain O2+? Is the O2+ ion paramagnetic?arrow_forwardDescribe the bonding in the O3 molecule and the NO2 ion, using the localized electron model. How would the molecular orbital model describe the bonding in these two species?arrow_forward
- The ionization energy of O2 is smaller than the ionization energy of atomic O; the opposite is true for the ionization energies of N2 and atomic N. Explain this behavior in terms of the molecular orbital energy diagrams of O2 and N2.arrow_forwardDraw the Lewis structures, predict the molecular structures, and describe the bonding (in terms of the hybrid orbitals for the central atom) for the following. a. XeO3 b. XeO4 c. XeOF4 d. XeOF2 e. XeO3F2arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





