
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 18, Problem 65IL
Titanium(IV) oxide is converted to titanium carbide with carbon at a high temperature.
TiO2(s) + 3 C(s) → 2 CO(g) + TiC(s)
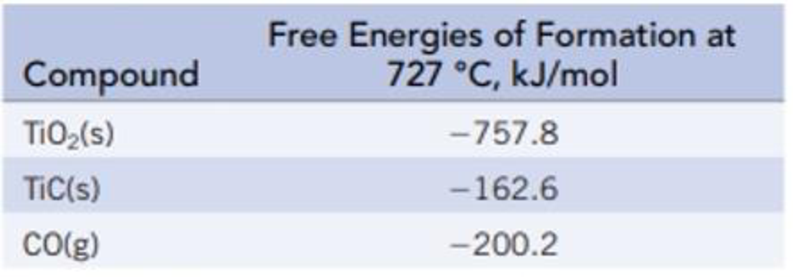
- (a) Calculate ΔrG° and K at 727 °C.
- (b) Is the reaction product-favored at equilibrium at this temperature?
- (c) How can the reactant or product concentrations be adjusted for the reaction to proceed at 727 °C?
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 18 Solutions
Chemistry & Chemical Reactivity
Ch. 18.1 - 1. A process is spontaneous in the direction that...Ch. 18.1 - A process that is reactant-favored at equilibrium...Ch. 18.1 - Prob. 3RCCh. 18.2 - In a spontaneous process, S(universe) is (a) 0 (b)...Ch. 18.2 - 2. Which of the following is true for a...Ch. 18.2 - Prob. 3RCCh. 18.3 - Prob. 1RCCh. 18.3 - Prob. 2RCCh. 18.4 - Predict which substance in each pair has the...Ch. 18.4 - Prob. 2CYU
Ch. 18.4 - Without looking up their standard entropies in...Ch. 18.4 - Without doing any calculations, predict the sign...Ch. 18.4 - Calculate rS for the following reaction at 25 C....Ch. 18.5 - Based on rH and rS, predict the spontaneity of the...Ch. 18.5 - Prob. 1RCCh. 18.5 - Prob. 2RCCh. 18.5 - Prob. 3RCCh. 18.6 - Prob. 1RCCh. 18.6 - Prob. 2RCCh. 18.7 - Prob. 1CYUCh. 18.7 - Prob. 2CYUCh. 18.7 - Oxygen was first prepared by Joseph Priestley...Ch. 18.7 - Prob. 4CYUCh. 18.7 - Prob. 5CYUCh. 18.7 - Prob. 6CYUCh. 18.7 - Prob. 1RCCh. 18.7 - Prob. 2RCCh. 18.7 - Prob. 3RCCh. 18.7 - Consider the hydrolysis reactions of creatine...Ch. 18.7 - Prob. 2QCh. 18.A - The decomposition of diamond to graphite...Ch. 18.A - It has been demonstrated that buckminsterfullerene...Ch. 18 - Which substance has the higher entropy? (a) dry...Ch. 18 - Which substance has the higher entropy? (a) a...Ch. 18 - Use S values to calculate the standard entropy...Ch. 18 - Use S values to calculate the standard entropy...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Calculate the standard entropy change for the...Ch. 18 - Is the reaction Si(s) + 2 Cl2(g) SiCl4(g)...Ch. 18 - Is the reaction Si(s) + 2 H2(g) SiH4(g)...Ch. 18 - Calculate S(universe) for the decomposition of 1...Ch. 18 - Calculate S(universe) for the formation of 1 mol...Ch. 18 - Classify each of the reactions according to one of...Ch. 18 - Classify each of the reactions according to one of...Ch. 18 - Using values of fH and S, calculate rG for each of...Ch. 18 - Using values of fH and S, calculate rG for each of...Ch. 18 - Using values of fH and S, calculate the standard...Ch. 18 - Using values of fH and S, calculate the standard...Ch. 18 - Using values of fG, calculate rG for each of the...Ch. 18 - Using values of fG, calculate rG for each of the...Ch. 18 - For the reaction BaCO3(s) BaO(s) + CO2(g), rG =...Ch. 18 - For the reaction TiCl2(s) + Cl2(g) TiCl4(), rG =...Ch. 18 - Determine whether the reactions listed below are...Ch. 18 - Determine whether the reactions listed below are...Ch. 18 - Heating some metal carbonates, among them...Ch. 18 - Calculate rH and rS for the reaction of tin(IV)...Ch. 18 - The standard free energy change, rG, for the...Ch. 18 - Prob. 28PSCh. 18 - Calculate rG at 25 C for the formation of 1.00 mol...Ch. 18 - Prob. 30PSCh. 18 - Prob. 31PSCh. 18 - Prob. 32PSCh. 18 - Compare the compounds in each set below and decide...Ch. 18 - Using standard entropy values, calculate rS for...Ch. 18 - About 5 billion kilograms of benzene, C6H6, are...Ch. 18 - Hydrogenation, the addition of hydrogen to an...Ch. 18 - Is the combustion of ethane, C2H6, product-favored...Ch. 18 - Prob. 38GQCh. 18 - When vapors from hydrochloric acid and aqueous...Ch. 18 - Calculate S(system), S(surroundings), and...Ch. 18 - Methanol is now widely used as a fuel in race...Ch. 18 - The enthalpy of vaporization of liquid diethyl...Ch. 18 - Calculate the entropy change, rS, for the...Ch. 18 - Using thermodynamic data, estimate the normal...Ch. 18 - Prob. 45GQCh. 18 - When calcium carbonate is heated strongly, CO2 gas...Ch. 18 - Sodium reacts violently with water according to...Ch. 18 - Yeast can produce ethanol by the fermentation of...Ch. 18 - Elemental boron, in the form of thin fibers, can...Ch. 18 - Prob. 50GQCh. 18 - Prob. 51GQCh. 18 - Estimate the boiling point of water in Denver,...Ch. 18 - The equilibrium constant for the butane ...Ch. 18 - A crucial reaction for the production of synthetic...Ch. 18 - Calculate rG for the decomposition of sulfur...Ch. 18 - Prob. 56GQCh. 18 - A cave in Mexico was recently discovered to have...Ch. 18 - Wet limestone is used to scrub SO2 gas from the...Ch. 18 - Sulfur undergoes a phase transition between 80 and...Ch. 18 - Calculate the entropy change for dissolving HCl...Ch. 18 - Some metal oxides can be decomposed to the metal...Ch. 18 - Prob. 62ILCh. 18 - Prob. 63ILCh. 18 - Prob. 64ILCh. 18 - Titanium(IV) oxide is converted to titanium...Ch. 18 - Cisplatin [cis-diamminedichloroplatinum(II)] is a...Ch. 18 - Prob. 67SCQCh. 18 - Explain why each of the following statements is...Ch. 18 - Decide whether each of the following statements is...Ch. 18 - Under what conditions is the entropy of a pure...Ch. 18 - Prob. 71SCQCh. 18 - Consider the formation of NO(g) from its elements....Ch. 18 - Prob. 73SCQCh. 18 - The normal melting point of benzene, C6H6, is 5.5...Ch. 18 - Prob. 75SCQCh. 18 - For each of the following processes, predict the...Ch. 18 - Heater Meals are food packages that contain their...Ch. 18 - Prob. 78SCQCh. 18 - Prob. 79SCQCh. 18 - Prob. 80SCQCh. 18 - Iodine, I2, dissolves readily in carbon...Ch. 18 - Prob. 82SCQCh. 18 - Prob. 83SCQCh. 18 - Prob. 84SCQCh. 18 - Prob. 85SCQCh. 18 - Prob. 86SCQCh. 18 - The Haber-Bosch process for the production of...Ch. 18 - Prob. 88SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the data in Appendix J to calculate rG andKPat 25 C for the reaction 2HBr(g)+Cl2(g)2HCl(g)+Br2() Comment on the connection between the sign of rG and the magnitude ofKP.arrow_forwardElemental boron, in the form of thin fibers, can be made by reducing a boron halide with H2. BCl3(g) + 3/2 H2(g) B(s) + 3HCl(g) Calculate H, S, and G at 25 C for this reaction. Is the reaction predicted to be product favored at equilibrium at 25 C? If so, is it enthalpy driven or entropy driven?arrow_forwardWhat is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the synthesis of ammonia? 3H2(g) + N2(g) 2NH3(g)arrow_forward
- Using values of fH and S, calculate rG for each of the following reactions at 25 C. (a) 2 Na(s) + 2 H2O() 2 NaOH(aq) + H2(g) (b) 6 C(graphite) + 3 H2(g) C6H6() Which of these reactions is (are) predicted to be product-favored at equilibrium? Are the reactions enthalpy- or entropy-driven?arrow_forwardYeast can produce ethanol by the fermentation of glucose (C6H12O6), which is the basis for the production of most alcoholic beverages. C6H12O6(aq) 2 C2H5OH() + 2 CO2(g) Calculate rH, rS, and rG for the reaction at 25 C. Is the reaction product- or reactant-favored at equilibrium? In addition to the thermodynamic values in Appendix L, you will need the following data for C6H12O6(aq): fH = 1260.0 kl/mol; S = 289 J/K mol; and fG = 918.8 kl/mol.arrow_forwardWhat is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the explosive decomposition of TNT? Use your knowledge of TNT and the chemical equation, particularly the phases, to answer this question. (Thermodynamic data for TNT are not in Appendix G.) 2C7H5N3O6(s) 3N2(g) + 5H2O() + 7C(s) + 7CO(g)arrow_forward
- a Calculate K1, at 25C for sulfurous acid: H2SO3(aq)H+(aq)+HSO3(aq) b Which thermodynamic factor is the most significant in accounting for the fact that sulfurous acid is a weak acid? Why?arrow_forwardCalculate the standard Gibbs free-energy change when SO3 forms from SO2 and O2 at 298 K. Why is sulfur trioxide an important substance to study? (Hint: What happens when it combines with water?)arrow_forwardConsider the decomposition of red mercury(II) oxide under standard state conditions.. 2HgO(s,red)2Hg(l)+O2(g) (a) Is the decomposition spontaneous under standard state conditions? (b) Above what temperature does the reaction become spontaneous?arrow_forward
- Chemists and engineers who design nuclear power plants have to worry about high-temperature reactions because it is possible for water to decompose. (a) Under what conditions does this reaction occur spontaneously? 2H2O(g) 2H2(g) + O2(g) (b) Under conditions where the decomposition of water is spontaneous, do nuclear engineers have to worry about an oxygen/hydrogen explosion? Justify your answer.arrow_forwardSilver carbonate, Ag2CO3, is a light yellow compound that decomposes when heated to give silver oxide and carbon dioxide: Ag2CO3(s)Ag2O(s)+CO2(g) A researcher measured the partial pressure of carbon dioxide over a sample of silver carbonate at 220C and found that it was 1.37 atm. Calculate the partial pressure of carbon dioxide at 25C. The standard enthalpies of formation of silver carbonate and silver oxide at 25C are 505.9 kJ/mol and 31.05 kJ/mol, respectively. Make any reasonable assumptions in your calculations. State the assumptions that you make, and note why you think they are reasonable.arrow_forwardUse the data in Appendix G to calculate the standard entropy change for H2(g) + CuO(s) H2O() + Cu(s)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY