
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
3rd Edition
ISBN: 9781259298424
Author: SMITH
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 18.6, Problem 18.19P
Interpretation Introduction
(a)
Interpretation:
The product formed when the following ammonium salt is treated with
Concept Introduction:
Amines are an organic compound that is derived from ammonia by replacing one or more hydrogen atoms of ammonia by the alkyl groups.- The ammonium ion has hydrogen atoms with a small positive charge. Therefore, the oxygen atom of the hydroxyl ion attacks the hydrogen atom. Then one N-H bond breaks and is bonded to hydroxyl ion to form a water molecule. Thus, the ammonium ion turns into the corresponding amine.
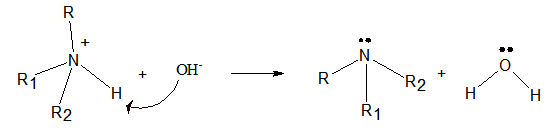
Interpretation Introduction
(b)
Interpretation:
The product formed when the following ammonium salt is treated with
Concept Introduction:
- Amines are organic compound which are derived from ammonia by replacing one or more hydrogen atoms of ammonia by the alkyl groups.
- The ammonium ion has hydrogen atoms with a small positive charge. Therefore, the oxygen atom of the hydroxyl ion attacks the hydrogen atom. Then one N-H bond breaks and is bonded to hydroxyl ion to form a water molecule. Thus, the ammonium ion turns into the corresponding amine.
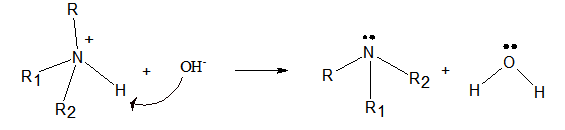
Interpretation Introduction
(c)
Interpretation:
The product formed when the following ammonium salt is treated with
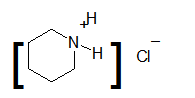
Concept Introduction:
- Amines are organic compound which are derived from ammonia by replacing one or more hydrogen atoms of ammonia by the alkyl groups.
- The ammonium ion has hydrogen atoms with a small positive charge. Therefore, the oxygen atom of the hydroxyl ion attacks the hydrogen atom. Then one N-H bond breaks and is bonded to hydroxyl ion to form a water molecule. Thus, the ammonium ion turns into the corresponding amine.
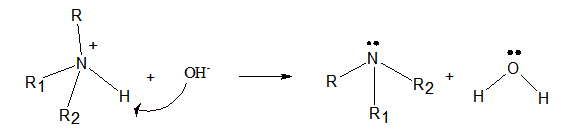
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
How does the reaction shown effect the water solubilty of the product?
H
HCI
dhe
A. product is more water soluble than the parent compound
B. product is less water soluble than the parent compound
Draw the products of each acid-base reaction.
H CH3
H CH,CH,NHCH3
COOH
+
а.
NAOH
b.
CF3
+
HCI
CH,O
naproxen
anti-inflammatory agent
fluoxetine
antidepressant
1
1
Show how to bring about each conversion in good yield.
a.
b.
C6H5
Cl
OH
COOH
C6H5 COOH
Chapter 18 Solutions
EBK GENERAL, ORGANIC, & BIOLOGICAL CHEM
Ch. 18.1 - Prob. 18.1PCh. 18.1 - Prob. 18.2PCh. 18.1 - Prob. 18.3PCh. 18.1 - Prob. 18.4PCh. 18.2 - Prob. 18.5PCh. 18.2 - Prob. 18.6PCh. 18.2 - Prob. 18.7PCh. 18.3 - Prob. 18.8PCh. 18.4 - Decaffeinated coffee is produced by extracting the...Ch. 18.4 - Prob. 18.10P
Ch. 18.5 - Prob. 18.11PCh. 18.5 - Prob. 18.12PCh. 18.5 - Prob. 18.13PCh. 18.6 - Prob. 18.14PCh. 18.6 - Prob. 18.15PCh. 18.6 - Prob. 18.16PCh. 18.6 - Name each ammonium salt. a. ( CH3 NH3)+Cl b. [( CH...Ch. 18.6 - Prob. 18.18PCh. 18.6 - Prob. 18.19PCh. 18.7 - Prob. 18.20PCh. 18.7 - Prob. 18.21PCh. 18.8 - Prob. 18.22PCh. 18.8 - Prob. 18.23PCh. 18.8 - Prob. 18.24PCh. 18.9 - Prob. 18.25PCh. 18.9 - Prob. 18.26PCh. 18.9 - Prob. 18.27PCh. 18.10 - Prob. 18.28PCh. 18 - Prob. 18.29PCh. 18 - Prob. 18.30PCh. 18 - Prob. 18.31PCh. 18 - Prob. 18.32PCh. 18 - Prob. 18.33PCh. 18 - Prob. 18.34PCh. 18 - Prob. 18.35PCh. 18 - Prob. 18.36PCh. 18 - Prob. 18.37PCh. 18 - Prob. 18.38PCh. 18 - Give an acceptable name for each amine. a. b.Ch. 18 - Give an acceptable name for each amine. a. b.Ch. 18 - Give an acceptable name for each amine. a. b. c....Ch. 18 - Give an acceptable name for each amine. a. CH3(...Ch. 18 - Prob. 18.43PCh. 18 - Prob. 18.44PCh. 18 - Prob. 18.45PCh. 18 - Prob. 18.46PCh. 18 - Prob. 18.47PCh. 18 - Prob. 18.48PCh. 18 - Prob. 18.49PCh. 18 - Prob. 18.50PCh. 18 - Which compound in each pair has the higher boiling...Ch. 18 - Which compound in each pair has the higher boiling...Ch. 18 - Draw the hydrogen-bonding interactions that occur...Ch. 18 - Prob. 18.54PCh. 18 - Prob. 18.55PCh. 18 - Which compound has the higher water solubility:...Ch. 18 - Prob. 18.57PCh. 18 - Prob. 18.58PCh. 18 - Draw the products of each acid-base reaction. a....Ch. 18 - Draw the products of each acid-base reaction. a....Ch. 18 - Prob. 18.61PCh. 18 - Prob. 18.62PCh. 18 - What type of nitrogen heterocycle occurs in both...Ch. 18 - Only one of the N atoms in nicotine has a trigonal...Ch. 18 - Prob. 18.65PCh. 18 - Prob. 18.66PCh. 18 - Why are aqueous solutions of an alkaloid slightly...Ch. 18 - Prob. 18.68PCh. 18 - Prob. 18.69PCh. 18 - Explain why patients with Parkinson’s disease...Ch. 18 - Prob. 18.71PCh. 18 - Prob. 18.72PCh. 18 - Prob. 18.73PCh. 18 - Prob. 18.74PCh. 18 - Prob. 18.75PCh. 18 - Prob. 18.76PCh. 18 - Prob. 18.77PCh. 18 - Prob. 18.78PCh. 18 - Prob. 18.79PCh. 18 - Prob. 18.80PCh. 18 - Prob. 18.81PCh. 18 - Prob. 18.82PCh. 18 - Prob. 18.83PCh. 18 - Prob. 18.84PCh. 18 - Prob. 18.85PCh. 18 - Prob. 18.86PCh. 18 - Prob. 18.87PCh. 18 - Why do some antihistamines cause drowsiness while...Ch. 18 - Prob. 18.89PCh. 18 - Prob. 18.90PCh. 18 - Compare the structures of morphine and heroin....Ch. 18 - Prob. 18.92CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the products of each acid-base reaction. NaOH b. + NazCO3 a. + CH3CH,CH, HO, OHarrow_forwardWhat happens when a carboxylic acid is treated with 1.PBr3 (or P),Br. 2.H2Oarrow_forwardDraw the product formed when phenylacetic acid (C6H5CH2COOH) is treated with each reagent. With some reagents, no reaction occurs. a. NaHCO3 b. NaOH c. SOCl2 d. NaCl e. NH3(1equiv) f. NH3, ∆ g. CH3OH, H2SO4 h. CH3OH, −OH i. [1] NaOH; [2] CH3COCl j. CH3NH2, DCC k. [1] SOCl2; [2] CH3CH2CH2NH2 (excess) l. [1] SOCl2; [2] (CH3)2; [2] (CHarrow_forward
- Compounds that contain both a hydroxyl group (OH) and a carboxyl group (COOH) can undergo an intramolecular esterifi cation reaction. What product is formed when each hydroxy acid undergoes an intramolecular reaction? a. HOCH 2CH 2CH 2CH 2CO 2H b. HOCH 2CH 2CH 2CO 2Harrow_forwardWhich of the following is the compound that when hydrolyzed produces these two products? X + H₂O* NH + H a. b. C. o Cr Ho C 0arrow_forwardWhat is the structure of compound A? 010 Compound A tot 01 Oll O III O IV OV || s ||| Compound A IV COOHarrow_forward
- what is the product? please show step by step? EtOH, + + H (1 eq) H¸CNH ,H 3 2 H G 1. EtOH, H* (1 eq) 2. H3CNH₂, H* Oarrow_forwardThe starting material that completes the following reaction is: NaOH, H₂O ? Draw Your Solution Harrow_forwardGive the product of the following reaction: a 'COOH COOH b COOH COOH KMnO4 но C COOH XX. COOH darrow_forward
- 1. What is the general formula for acyl halides? 2. List these acyl halides in terms of reactivity in increasing order. of H3C Br CH3 H,C CIarrow_forwardBr NaoH NH2 H>N (B HYO what is compound A and B?arrow_forwardRank the given compounds in order of decreasing basicity. 1=most basic, 4=least basicarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY