
Concept explainers
(a)
Interpretation:
The product of the given reduction reaction including stereochemistry is to be shown.
Concept Introduction:
The compound
Answer:
The product of the given reduction reaction including stereochemistry is shown below.
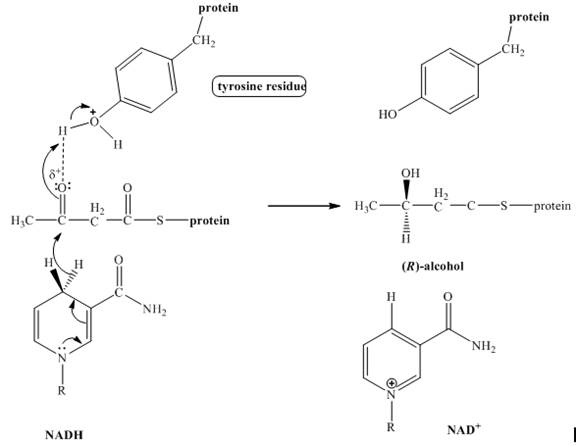
Explanation:
The enzyme
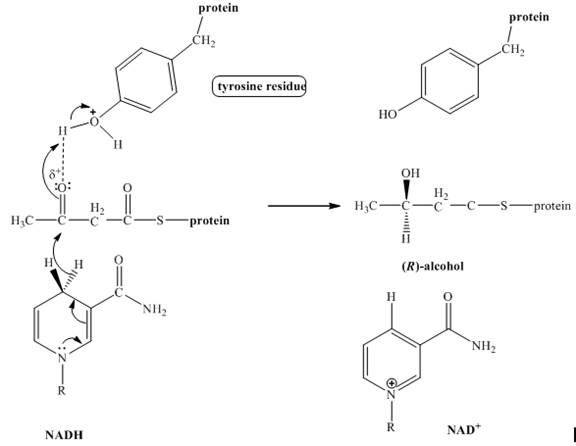
Figure 1
Conclusion:
The product formed in the given reaction including stereochemistry is shown in Figure 1.
(b)
Interpretation:
The relative positions of
Concept Introduction:
The compound
(c)
Interpretation:
The role of the side chain hydroxyl group of tyrosine and serine in this reaction is to be stated.
Concept Introduction:
The compound
Want to see the full answer?
Check out a sample textbook solution
Chapter 19 Solutions
Organic Chemistry
- An optically active monoterpene (compound A) with molecular formula C10H18O undergoes catalytic hydrogenation to form an optically inactive compound with molecular formula C10H20O (compound B). When compound B is heated with acid, followed by reaction with O3 and then with dimethyl sulfide, one of the products obtained is 4-methylcyclohexanone. Give possible structures for compounds A and B.arrow_forwardWhen 3-iodo-2,2-dimethylbutane is treated with silver nitrate in ethanol, three elimination products are formed. Give their structures, and predict which ones are formed inlarger amounts.arrow_forwardThe first step in the metabolism of glycerol, formed by digestion of fats, is phosphorylation of the pro-R—CH2OH group by reaction with adenosine triphosphate (ATP) to give the corresponding glycerol phosphate plus adenosine diphosphate (ADP). Show the stereochemistry of the product.arrow_forward
- Following are the steps in the industrial synthesis of glycerin. Provide structures for all intermediate compounds (AD) and describe the type of mechanism by which each is formed.arrow_forwardDHA is a fatty acid derived from sh oil and an abundant fatty acid in vertebrate brains. Hydrogenation of DHA forms docosanoic acid [CH3(CH2)20CO2H] and ozonolysis forms CH3CH2CHO, CH2(CHO)2 (ve equivalents), and HCOCH2CH2CO2H. What is the structure of DHA if all double bonds have the Z conguration?arrow_forwardCompound A, C11H16O, was found to be an optically active alcohol. Despite its apparent unsaturation, no hydrogen was absorbed on catalytic reduction over a Pd/C catalyst. On treatment of A with dilute H2SO4, dehydration occurred and an optically inactive alkene B, C11H14 was produced as the major product. Alkene B, on ozonolysis, gave two products. Product C, C8H8O, was shown to be a methyl ketone while product D, C3H6O, was shown to be an aldehyde.arrow_forward
- When pent-2-yne reacts with mercuric sulfate in dilute sulfuric acid, the product is amixture of two ketones. Give the structures of these products, and use mechanisms toshow how they are formedarrow_forwardCompound A, C11H16O, was found to be an optically active alcohol. Despite its apparent unsaturation, no hydrogen was absorbed on catalytic reduction over a Pd/C catalyst. On treatment of A with dilute H2SO4, dehydration occurred and an optically inactive alkene B, C11H14 was produced as the major product. Alkene B, on ozonolysis, gave two products. Product C, C7H6O, was shown to be an aldehyde while product D, C4H8O, was shown to be a ketone. Draw the structure of compound C. You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. In cases where there is more than one answer, just draw one. HELP PLEASE I DONT UNDERSTAND THE PROCESSarrow_forward4.4 Show how the compound thiodiazine - C21H26N2S2 - decomposes anaerobically and how these end products in turn decompose aerobically to stabilized sulfurand nitrogen compounds.arrow_forward
- Isoerythrogenic acid, C18H26O2, is a acetylic fatty acid that turns vivid vle on exposure to UV light. On Catalytic hydrogenation over a palladium catalyst, five molar equivalents of hydrogen are absorbed, and stearic acidarrow_forwardA difficult problem in the synthesis of PGF2α is the introduction of the OH group at C15 in the desired conguration.a. Label this stereogenic center as R or S.b. A well known synthesis of PGF2α involves reaction of A with Zn(BH4)2, a metal hydride reagent similar in reactivity to NaBH4, to form two isomeric products, B and C. Draw their structures and indicate their stereochemical relationship.c. Suggest a reagent to convert A to the single stereoisomer X.arrow_forwardA difficult problem in the synthesis of PGF2α is the introduction of the OH group at C15 in the desired configuration. a. Label this stereogenic center as R or S. b. A well known synthesis of PGF2α involves reaction of A with Zn(BH4)2, a metal hydride reagent similar in reactivity to NaBH4, to form two isomeric products, B and C. Draw their structures and indicate their stereochemical relationship. c. Suggest a reagent to convert A to the single stereoisomer X.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

