
Concept explainers
Give the symbol showing the
- a. 9 protons and 10 neutrons (used in nuclear medicine).
- b. 26 protons and 30 neutrons (the most stable isotope of this element).
- c. 86 protons and 136 neutrons (the radioactive gas found in some homes).
(a)
Interpretation:
The symbol has to be given for the isotope which has 9 protons and 10 neutrons.
Concept Introduction:
According to modern periodic table, both the physical and chemical properties of elements depend on their atomic number in a periodic way.
Modern periodic table contains groups and periods where the elements same number of outer electrons comes under same group and elements having same number of electron shells are in same period.
Figure 1
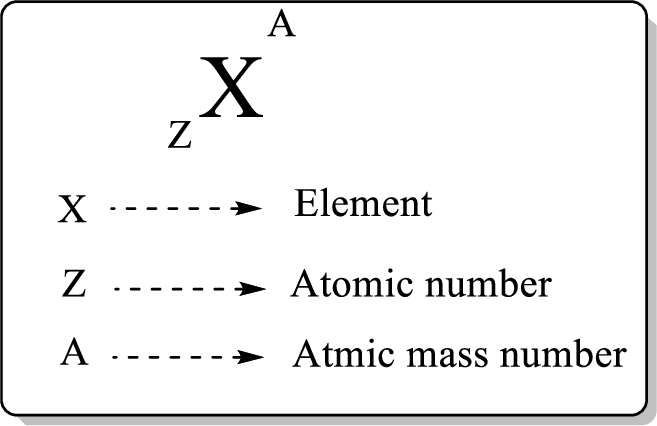
In a periodic table, an element is represented as shown below,
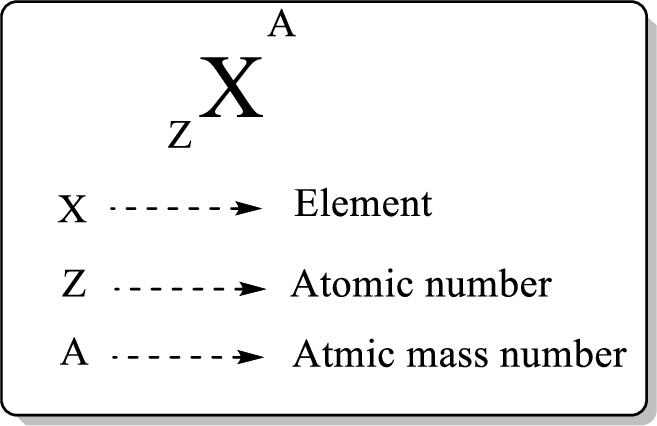
Atomic number is equal to the number of proton (
The atomic mass can be calculated using the formula,
Isotopes are species which have the same number of protons and different mass number.
Examples:
Explanation of Solution
The given isotope has 9 protons and 10 neutrons. Proton number helps to find the isotope’s symbol.
The element which has nine protons is Flourine and its symbol is F.
Mass number of the isotope can be determined by using the equation,
Thus, the isotope can be represented using its symbol as follows,
(b)
Interpretation:
The symbol has to be given for the isotope which has 26 protons and 30 neutrons.
Concept Introduction:
According to modern periodic table, both the physical and chemical properties of elements depend on their atomic number in a periodic way.
Modern periodic table contains groups and periods where the elements same number of outer electrons comes under same group and elements having same number of electron shells are in same period.
Figure 1
In a periodic table, an element is represented as shown below,
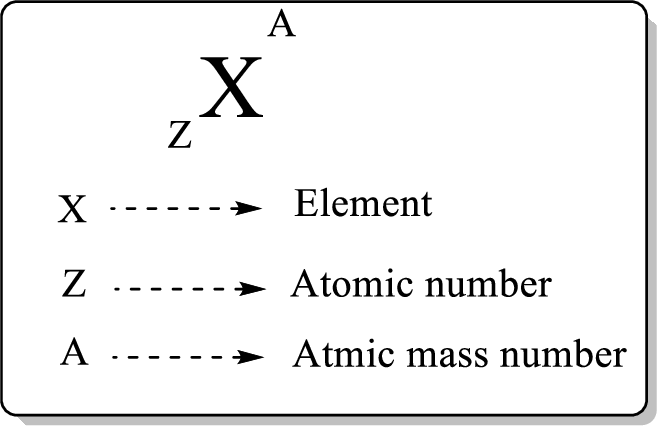
Atomic number is equal to the number of proton (
The atomic mass can be calculated using the formula,
Isotopes are species which have the same number of protons and different mass number.
Examples:
Explanation of Solution
The given isotope has 26 protons and 30 neutrons. Proton number helps to find the isotope’s symbol.
The element which has 26 protons is Iron and its symbol is Fe.
Mass number of the isotope can be determined by using the equation,
Thus, the isotope can be represented using its symbol as follows,
(c)
Interpretation:
The symbol has to be given for the isotope which has 86 protons and 136 neutrons.
Concept Introduction:
According to modern periodic table, both the physical and chemical properties of elements depend on their atomic number in a periodic way.
Modern periodic table contains groups and periods where the elements same number of outer electrons comes under same group and elements having same number of electron shells are in same period.
Figure 1
In a periodic table, an element is represented as shown below,
Atomic number is equal to the number of proton (
The atomic mass can be calculated using the formula,
Isotopes are species which have the same number of protons and different mass number.
Examples:
Explanation of Solution
The given isotope has 86 protons and 136 neutrons. Proton number helps to find the isotope’s symbol.
The element which has 86 protons is Radon and its symbol is Rn.
Mass number of the isotope can be determined by using the equation,
Thus, the isotope can be represented using its symbol as follows,
Want to see more full solutions like this?
Chapter 2 Solutions
Combo: Loose Leaf Chemistry In Context With Connect Access Card
Additional Science Textbook Solutions
Chemistry & Chemical Reactivity
Chemistry: A Molecular Approach
Basic Chemistry (5th Edition)
Organic Chemistry (9th Edition)
Living By Chemistry: First Edition Textbook
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
- The element silver (Ag) has two naturally occurring isotopes: 109 Ag and 107Ag with a mass of 106.905 u. Silver consists of 51.82% 107Ag and has an average atomic mass of 107.868 u. Calculate the mass of 109Ag.arrow_forwardA semiconducting material is composed of 52 g of Ga, 9.5 g of Al, and 112 g of As. Which element has the largest number of atoms in this material?arrow_forwardChlorine has two prominent isotopes,37Cl and35Cl . Which is more abundant? How do you know?arrow_forward
- How many platinum atoms are in a pure platinum ring weighing 4.32 g?arrow_forwardChlorine has two natural isotopes: 1737Cl and 1735Cl. Hydrogen reacts with chlorine to form the compound HCl. Would a given amount of hydrogen react with different masses of the two chlorine isotopes? Does this conflict with the law of definite proportion? Why or why not?arrow_forwardRadon is a radioactive gas that can cause lung cancer. It has been detected in the basement of some homes. In an atom of Rn-220, (a) how many protons are there? (b) how many neutrons are there?arrow_forward
- Uranium-235 is the isotope of uranium commonly used in nuclear power plants. How many (a) protons are in its nucleus? (b) neutrons are in its nucleus? (c) electrons are in a uranium atom?arrow_forwardMatch these by placing the correct notation in the appropriate blank. 3467Se3367As3567Br3672Kr a. Contains 33 neutrons b. Contains greatest number of neutrons c. Contains equal number of protons and neutrons d. Contains the same number of neutrons as there are protons in As-67arrow_forwardThe CRC Handbook, a large reference book of chemical and physical data, lists two isotopes of rubidium (Z=37). The atomic mass of 72.15 of rubidium atoms is 84.9118u. Through a typographical oversight, the atomic mass of the second isotope is not printed. Calculate that atomic mass.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





