
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
9th Edition
ISBN: 9780137249442
Author: Wade
Publisher: INTER PEAR
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 2, Problem 2.35SP
Predict which compound in each pair has the higher boiling point. Explain your prediction.
- a. CH3CH2OCH3 or CH3CH(OH)CH3
- b. CH3CH2CH2CH3 or CH3CH2CH2CH2CH3
- c. CH3CH2CH2CH2CH3 or (CH3)2CHCH2CH3
- d. CH3CH2CH2CH2CH3 or CH3CH2CH2CH2CH2Cl
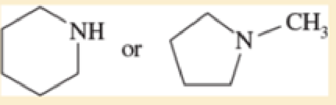
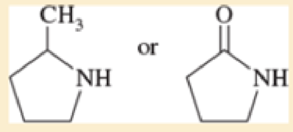
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Part A
Arrange the following in order of increasing boiling point: CH4, CH3CH2CH3, CH3CH2CH2CI, CH3CH2CH2OH.
Rank from smallest to largest. To rank items as equivalent, overlap them.
Reset Help
CH3CH2CH2C1
CH3CH2CH2OH
CH4
CH3CH2CH3
lowest boiling point
highest boiling point
In each pair of compounds, pick the one with the higher boiling point. Explain your reasoning.a. CH3OH or CH3SHb. CH3OCH3 or CH3CH2OHc. CH4 or CH3CH3
a. Which of the following compounds forms hydrogen bonds between its molecules? 1. CH3CH2OCH2CH2OH 2. CH3CH2N(CH3)2 3. CH3CH2CH2CH2Br 4. CH3CH2CH2NHCH3 5. CH3CH2CH2COOH 6. CH3CH2CH2CH2F b. Which of the preceding compounds forms hydrogen bonds with a solvent such as ethanol?
Chapter 2 Solutions
ORGANIC CHEMISTRY MASTERINGCHEM ACCESS
Ch. 2.1A - Prob. 2.1PCh. 2.1B - The NF bond is more polar than the NH bond: but...Ch. 2.1B - For each of the following compounds 1. Draw the...Ch. 2.1B - Two isomers of 1,2-dichloroethene are known One...Ch. 2.2C - Prob. 2.5PCh. 2.2C - Prob. 2.6PCh. 2.3 - Prob. 2.7PCh. 2.4 - Calculate the pH of the following solutions a....Ch. 2.6A - Ammonia appears in Table 2-2 as both an acid and a...Ch. 2.7 - Write equations for the following acid-base...
Ch. 2.7 - Ethanol, methylamine. and acetic acid are all...Ch. 2.8 - Prob. 2.12PCh. 2.10 - Write equations for the following acid-base...Ch. 2.10 - Rank the following acids in decreasing order of...Ch. 2.11 - Prob. 2.15PCh. 2.11 - Prob. 2.16PCh. 2.11 - Consider each pair of bases and explain which one...Ch. 2.12 - Which is a stronger base ethoxide ion or acetate...Ch. 2.12 - Prob. 2.19PCh. 2.12 - Prob. 2.20PCh. 2.12 - Prob. 2.21PCh. 2.12 - Choose the more basic member of each pair of...Ch. 2.14 - Prob. 2.23PCh. 2.15D - Classify the following hydrocarbons and draw a...Ch. 2.16D - Prob. 2.25PCh. 2.17C - Draw a Lewis structure and classify each of the...Ch. 2.17C - Circle the functional groups in the following...Ch. 2 - The CN triple bond in acetonitrile has a dipole...Ch. 2 - Prob. 2.29SPCh. 2 - Sulfur dioxide has a dipole moment of 1.60 D....Ch. 2 - Which of the following pure compounds can form...Ch. 2 - Predict which member of each pair is more soluble...Ch. 2 - Prob. 2.33SPCh. 2 - Prob. 2.34SPCh. 2 - Predict which compound in each pair has the higher...Ch. 2 - All of the following compounds can react as acids...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - Rank the following species in order of increasing...Ch. 2 - The Ka of phenylacetic acid is 5 2 105, and the...Ch. 2 - The following compound can become protonated on...Ch. 2 - The following compounds are listed in increasing...Ch. 2 - Prob. 2.42SPCh. 2 - Prob. 2.43SPCh. 2 - Compare the relative acidity of 1-molar aqueous...Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - The following compounds can all react as bases. a....Ch. 2 - The following compounds can all react as acids. a....Ch. 2 - Prob. 2.48SPCh. 2 - Methyllithium (CH3Li) is often used as a base in...Ch. 2 - Label the reactants in these acid-base reactions...Ch. 2 - In each reaction, label the reactants as Lewis...Ch. 2 - Prob. 2.52SPCh. 2 - Each of these compounds can react as a nucleophile...Ch. 2 - Prob. 2.54SPCh. 2 - Give a definition and an example for each class of...Ch. 2 - Circle the functional groups in the following...Ch. 2 - Prob. 2.57SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Rank the following in terms of increasing boiling point: CH3CH2OCH2CH3 CH3CH2OCH3 CH3CH2CH2CH3 CH3CH2CH2CH2NH2 a. CH3CH2OCH3 I >I В)I > I > I C) I > II > III D) II > Ш >I E) II > I > П 3. The normal boiling point for H Br is higher than the normal boiling point for H Cl. This can be explained by A) larger dipole-dipole forces for H Br . B) larger dispersion forces for H Br . C) larger hydrogen-bond forces for H Br. D) larger dipole-dipole forces, larger dispersion forces, and larger hydrogen-bond forces for H Br . 4. Place the following substances in order of increasing boiling point. А) Не < I2 < 02 B) I2 < 02 < He С) 02 < I2 < Не D) I2 < He < 02 E) He < 02 < I2arrow_forwardWhich has higher boiling point? A. CH3CH2CH2CH2CH3 B. (CH3)2CHCH2CH3arrow_forward3) Identify the compound with the lowest boiling point. A. CH3CH2CH2OH B. CH:CH(OH)CH3 C. CH3OCH2CH3 D. CH3CH2CH2CH2OH E. CH3CH2OCH2CH3arrow_forward
- In each pair of compounds, pick the one with the higher vaporpressure at a given temperature. Explain your reasoning.a. CH4 or CH3Cl b. CH3CH2CH2OH or CH3OHc. CH3OH or H2COarrow_forwardSelect the compound with the highest boiling point. I CH3CH₂CH₂CH₂NH₂ A) I B) II II CH3CH₂NHCH₂CH3 C) III III CH3CH₂NCH3 CH3arrow_forwardArrange these compounds in order of increasing boiling point. Explain your reasoning. a. CH, b. CH,CH, c. CH;CH2CI d. CH,CH;OHarrow_forward
- Which molecule will have a higher boiling point and why a. molecule ‘b’ will have a higher boiling point as the molecule is more compact and therefore will pack better. b. molecule ‘b’ will have the higher boiling point as the methyl groups are tetrahedrally arranged around the central carbon, giving it higher symmetry. c. molecule ‘a’ will have a higher boiling point as it has a greater surface for London dispersion forces. d. molecule ‘a’ will have a higher boiling point as it has a higher molecular weight than molecule ‘b’.arrow_forwardCompound A has a boiling point of 200 and compound b has a boiling point of 115. Which is a higher safety concern?arrow_forwardRank the compounds in each group in order of increasing boiling point. a. CH3(CH2)4I, CH3(CH2)sI, CH3(CH)gI b. CH;CH,CH,NH2, (CH3);N, CH3CH2CH,CH3 c. (CHa),COC(CH3)3, CHa(CH2)30(CH)¿CH3, CH3(CH2);OH COH d. HO. Br е. OH f.arrow_forward
- 2. Which one of the following compounds would be expected to have the highest normal boiling point? a) CH3CH2CH3 b) CH3CH2CH2CH2CH3 c) CH3CH2CH2CH2CH2CH3 d) CH3CH2CH2CH2CH2CH2CH3 Carrow_forwardCH3ch2ch2ch2ch2Oh Boiling-oing lowest, intermediate, highest boiling point ch2ch2ch2ch2ch2ch3 ch3ch2ch2ch2ch2ch3arrow_forwardWhich compound in the following pairs will have the higher boiling point? Explain your reasoning. a. NH3 or PH3 b. ethylene glycol (HOCH2CH2OH) or ethanol c. 2,2-dimethylpropanol [CH3C(CH3)2CH2OH] or n-butanol (CH3CH2CH2CH2OH)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY