
(a)
Interpretation:
The plot of ln y versus x on rectangular coordinates passes through (1.0, 693) and (2,0) should be drawn.
Concept introduction:
The straight-line plot has following equation:
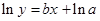
Here, 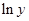 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 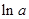 is intercept.
is intercept.
And, the following equation shows the exponential plot:
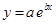
(b)
Interpretation:
The semilog plot of y versus x passes through (1,2) and (2,1) should be drawn.
Concept introduction:
The straight-line plot has following equation:
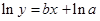
Here, 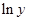 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 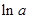 is intercept.
is intercept.
And, the following equation shows the exponential plot:
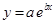
(c)
Interpretation:
A log pot y versus x passes through (1,2) and (2,1), determine the equation.
Concept introduction:
The straight-line plot has following equation:
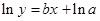
Here, 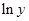 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 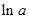 is intercept.
is intercept.
And, the following equation shows the exponential plot:
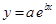
(d)
Interpretation:
A semilog plot of xy (logarithmic axis) versus y/x passes through (1.0. 40.2). Determine the equation.
Concept introduction:
The straight-line plot has following equation:
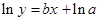
Here, 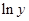 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 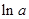 is intercept.
is intercept.
And, the following equation shows the exponential plot:
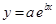
(e)
Interpretation:
The equation of log plot of y2/x versus (x-2) passes through (1.0, 40.2) and (2.0, 807.0)
Concept introduction:
The straight-line plot has following equation:
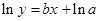
Here, 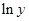 values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and
values are plotted at y axis, x values are plotted at x axis, b is slope of the reaction and 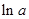 is intercept.
is intercept.
And, the following equation shows the exponential plot:
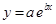
Trending nowThis is a popular solution!

Chapter 2 Solutions
EBK ELEMENTARY PRINCIPLES OF CHEMICAL P
- 2. Set the two equations equal and isolate In Ksp on one side of the equals sign by dividing both sides by (-RT). AG=RT'In Ksp=AH-TAS/-RT AG=In KSR=AH-TAS/-RT| 3. Create a graph using Excel plotting In Ksp on the y-axis and 1/T (make sure the units for temperature are Kelvin) on the x-axis. I In KSP 0 0.0029 -0.5 -1 -1.5 -2 -2.5 -3 -3.5 -4 -4.5 -5 0.003 0.0031 0.0032 1/T 0.0033 0.0034 0.0035 y=-2738x+5.075 R² = 0.9845 0.0036 a. Use the value obtained for the slope of the graph to solve for AH° using the equation derived in Question 2.arrow_forwardIn a survey of 1000 large corporations, 250 said that, given a choice between a job candidate who smokes and an equally qualified nonsmoker, the nonsmoker would get the job (USA Today).(a) Let p represent the proportion of all corporations preferring a nonsmoking candidate. Find a point estimate for p.(b) Find a 0.95 confidence interval for p.(c) As a news writer, how would you report the survey results regarding the proportion of corporations that hire the equally qualified nonsmoker? What is the margin of error based on a 95% confidence interval?arrow_forwardUse the References to access important values if needed for this question. You have the following data points which belong to a function of the form y = ae", where b can be positive or negative. 4.13 3.26 2.57 0.100 0.200 0.300 You wish to determine the value of the constant, 6, and to do so you will construct a linear plot. What will you plot against what? y-axis label: Iny - x-axis label: x From the values of a and y in the table above, calculate the values of the three corresponding data points. Click on the graph below, and use the "Freeform Curves" tool to plot the data. Line segments will automatically connect the points. Enter Solution What is the numerical value of the constant, b? b = Submit Answer Retry Entire Group No more group attempts remainarrow_forward
- Part B Perform the same calculation for N2 under identical conditions. (Hint: Do you need to reevaluate the full expression for qT ?) Express your answer to three significant figures. ? qT (N2) = Submit Request Answerarrow_forward4 A model for the compound butane is shown. E 20 F3 D C Submit Answer 4 Write a balanced chemical equation that shows the formation of butane from the elements carbon (C) and hydrogen (H₂ ). (Use the smallest integer coefficients possible and do not include states.) ball & stick-+ labels 200 000 F4 R F + H % 5 V WH [Review Topics] [References] Use the References to access important values if needed for this question. Retry Entire Group 9 more group attempts remaining Cengage Learning Cengage Technical Support F5 T G B MacBook Air F6 Y H & 7 F7 U N * 8 J F8 M ( 9 K DD FO O F12 I } deletearrow_forwardBe sure to answer all parts. Enter your answer in scientific notation. Carry out the following calculation, paying special attention to significant figures, rounding, and units (J-joule, the SI unit of energy; mol = mole, the SI unit for amount of substance): (6.022 x 1023 atoms/mol) (4.68 x 10-18 J/atom)(1-2-3-2)=[25.051 Note that the numbers 1 and 3 in the last term are exact. x 10 J/molarrow_forward
- Chemistry HW need help. How to do these calculation base on the data that is given?arrow_forward3.58 4.42 5.48 0.100 0.200 0.300 You wish to determine the value of the constant, 6, and to do so you will construct a linear plot. What will you plot ag what? y-axis label: x-axis label: From the values of x and y in the table above, calculate the values of the three corresponding data points. Click on the graph below, and use th "Freeform Curves" tool to plot the data. Line segments will automatically connect the points. Enter Solution What is the numerical value of the constant, b? Previous b =arrow_forwardBalance each of the chemical equations shown below. Be sure to include values of 1 for any species that only have 1 equivalent involved in the reaction (i.e. empty inputs DO NOT correspond to the value "1"). Use integers for ALL coefficients.arrow_forward
- See image below. Can you please show me how to get to 0.00018 as the answer?arrow_forwardO cedarcrest.instructure.com b Math Exercise Sheet.docx Lab Math Exercise Sheet.docx 103 Lab Math Exercise Sheet.docx (15 KB) Page < 2 of 2 6) Given the equation P2V2 n,T, » rearrange it to solve for P, n,Tarrow_forwardor lons (1).uock mat Tools Help E Q Q Normal Times New ... BIU A A- 12 A student was given an unknown solid that gave the following test results: A portion of the solution of unknown emitted bubbles of a colorless, odorless gas when hydrochloric acid solution was added. Another portion of the solution gave a blood-red colored solution with potassium thiocyanate solution. 4. a) Which ions are present? Cation Anion b) Name the unknown salt. c) Write the correct formula for the salt. Another student's unknown gave the following test results: a portion of the solution of unknown gave a yellow precipitate with ammonium molybdate reagent. A flame test on some of the solution resulted in a bright yellow flame. 5. a) Which ions are present? Cation Anion b) Name the unknown salt. c) Write the correct formula for the salt. MO huluarrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning



