
(a)
Interpretation:
The expected ground-state electron configuration of
Concept Introduction:
The fundamental principles that are followed to write an electronic configuration include three rules as follows:
Electron in a
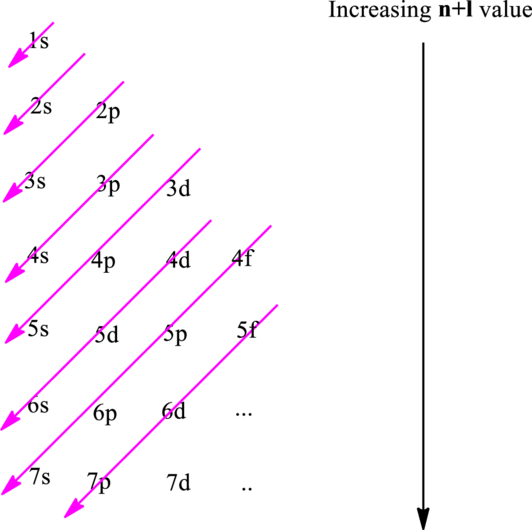
Hund’s rule suggests electrons are not allowed to be paired up until each degenerate set of orbital has got at least one electron.
Pauli Exclusion Principle states two electrons within the same orbital cannot possess same set for four possible quantum numbers.
In
The convention followed to remove or add electrons is electrons of largest principal quantum number are lost first. In case of subshells of the same
(b)
Interpretation:
The expected ground-state electron configuration of
Concept Introduction:
Refer to part (a).
(c)
Interpretation:
The expected ground-state electron configuration of
Concept Introduction:
Refer to part (a).
(d)
Interpretation:
The ground-state electronic configuration of
Concept Introduction:
Refer to part (a).
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
CHEM PRINCIPLES LL W/ACHIEVE ONE-SEM
- Nitrogen ions tend to have a charge of 3-; explain the reason for this pattern/trend.arrow_forwardFor many years after they were discovered, it was believed that the noble gases could not form compounds. Now we know that belief to be incorrect. A mixture of xenon and fluorine gases, confined in a quartz bulb and placed on a windowsill, is found to slowly produce a white solid. Analysis of the compound indicates that it contains 77.55% Xe and 22.45% F by mass.(a) What is the formula of the compound?(b) Write a Lewis structure for the compound.(c) Predict the shape of the molecules of the compound.(d) What hybridization is consistent with the shape you predicted?arrow_forward4. Write an appropriate set of four quantum numbers (n, l, ml & ms) that could be representative of a valence electron in each of the following atoms or ions. (a) Bi (m (b) Sr (c) Mo (d) Ru2+ (e) Euarrow_forward
- Write the electron configurations for: Calcium ion in CaCl₂:arrow_forwardMixing SbCl3 and GaCl3 in a 1;1 molar ratio (usingliquid sulfur dioxide as a solvent) gives a solid ioniccompound of empirical formula GaSbCl6. A controversyarises over whether this compound is ( ) SbCl2 + ( ) GaCl 4 - or( ) GaCl+ 2 ( ) SbCl 4 -(a) Predict the molecular structures of the two anions.(b) It is learned that the cation in the compound has abent structure. Based on this fact, which formulationis more likely to be correct?arrow_forwardIf an element is bonded to 4 other atoms and has a formal charge of +1, what group must the element be in? I know that group 3A atoms are elctron deficient, and that period 3 elements and below, except for group 3A elements like Aluminum, can expand their octet because of their available d-orbital, which may not be relevant to this problem. I don't understand this question, or why the answer would be 5A. Is it because 5A have odd valence electrons, and can form free radicals, like NO?arrow_forward
- The Pauling electronegativity of Cl is 3.2 while that of Br is 3.0. Estimate the bond dissociation energy of D(CI-Br) bond in kJ/mol. (given: D(Br-Br)=158 kJ/mol, D(CI- CI)=242 kJ/mol.) (1.0 eV = 96.5 kJ/mol). O 204 196 219 228 O 237arrow_forwardDetermine the average Cl-F bond energy, in units of kJ mol¯, in CIF5, using t following data: • Cl2(g) + 5 F2(g) → 2 CIF5(g) A,H° = -477 kJ mol-1 159 kJ mol-1 • bond energy F-F: • bond energy Cl-Cl: 243 kJ mol-1arrow_forwardWrite electron configurations for the following ions. Ru3+ Pd2+ Au3+arrow_forward
- Two fourth-period atoms, one of a transition metal, M, and the other of a main-group nonmetal, X, form a compound with the formula M2X3. What is the electron configuration of atom X if M is Fe? What is the configuration of X if M is Co, if X is the same then give the full electron configuration for the Co ion.arrow_forwardIron(III) sulfate [Fe2(SO4)3] is composed of Fe3+ and SO4 2− ions. Explain why a sample of iron(III) sulfate is uncharged.arrow_forwardGive a reason why each of the following statements is a safeprediction:(a) Every compound of Rb with a nonmetal is ionic in character.(b) Every compound of nitrogen with a halogen element is amolecular compound.(c) The compound MgKr2 does not exist.(d) Na and K are very similar in the compounds they form withnonmetals.(e) If contained in an ionic compound, calcium (Ca) will be inthe form of the doubly charged ion, Ca2arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
