
Label each region on the periodic table.
- Noble gases
- Period 3
- Group 4A
- s block elements
- alkaline earth elements
- f block elements
transition metals - group 10
(a)
Interpretation:
Theregion of noble gases on the periodic table is to be labeled.
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group.
Answer to Problem 33P
The noble gases are placed on the extreme right column of the periodic table as shown below.
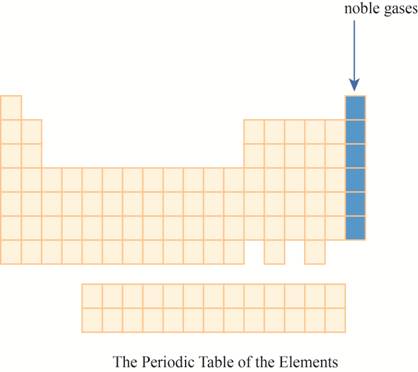
Explanation of Solution
Theelements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell.
The noble gases have their outermost shell fully filled with electrons.Due to fully filled outer atomic orbital, they get the place on extreme right of the periodic table.
The region of noble gases on the periodic table is shown below.
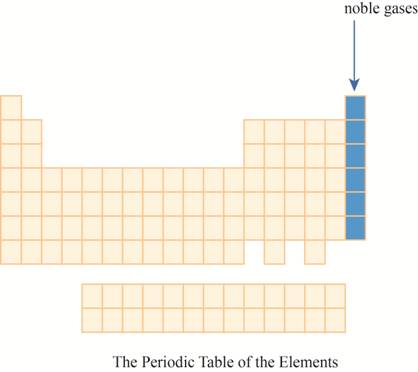
Figure 1
(b)
Interpretation:
The region of period
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group.
Answer to Problem 33P
The period
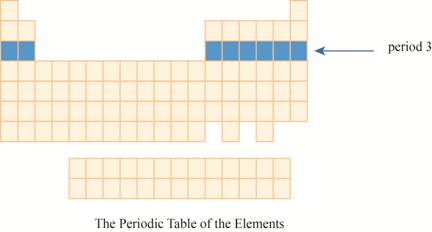
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled, the next atom goes to the next row or period.
The third row of the periodic table is known as third period. As the electron goes into third energy level or shell, they are placed in third row. The third shell has two orbitals
These elements are (sodium on the extreme left) to argon (on the extreme right) with atomic numbers,
The region of period
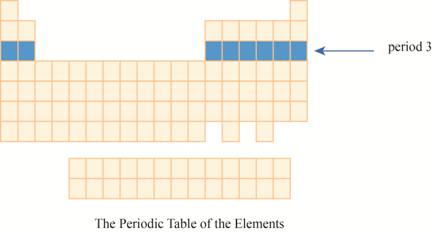
Figure 2
(c)
Interpretation:
The region of group
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group
Answer to Problem 33P
The group
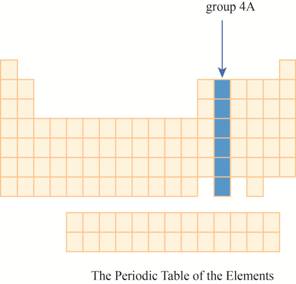
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
The transition metals are given place in the middle of the periodic table and to its right, main group elements having valence electrons
The group
It has main group elements, carbon and its family. The region of group
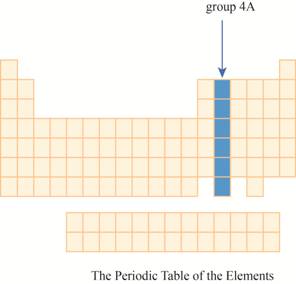
Figure 3
(d)
Interpretation:
The region of
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in an increasing order of the atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group
Answer to Problem 33P
The
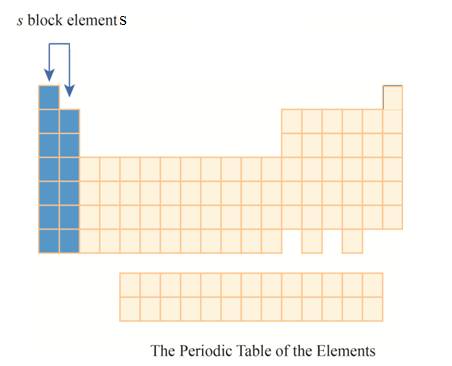
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
The
The first column has elements with one electron in their outer
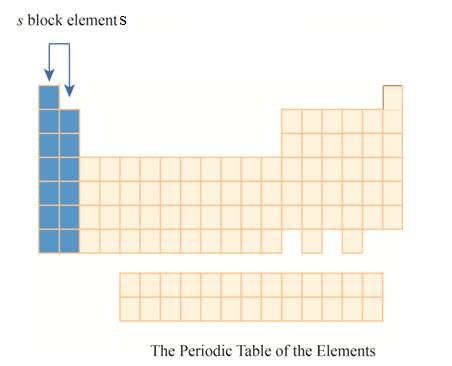
Figure 4
(e)
Interpretation:
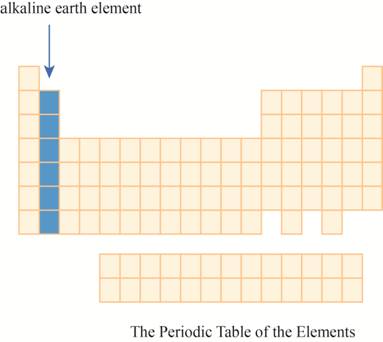
The region of alkaline earth elements on the periodic table is to be labeled.
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group
Answer to Problem 33P
The alkaline earth elements are kept in first two columns on the extreme left of periodic tableas shown below.
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
These elements have two electrons in their outer
The alkaline earth elements are placed in the second columns on the left of periodic table. The elements of this column has electron in their outer
The region of alkaline earth elements on the periodic table are shown below.
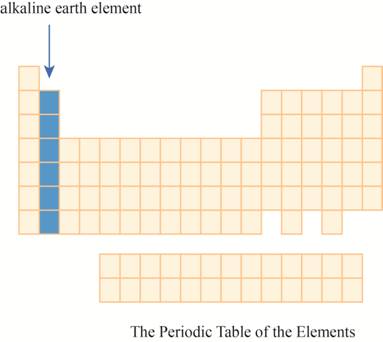
Figure 5
(f)
Interpretation:
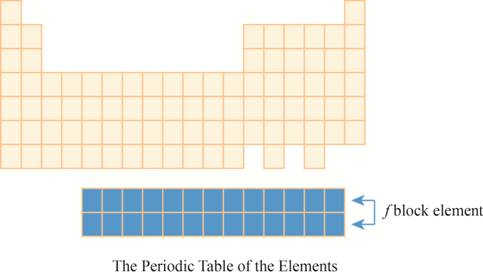
The region of
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group
Answer to Problem 33P
The
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
The
The placement of these elements below the main periodic table is due to their electronic configuration. The
The region of
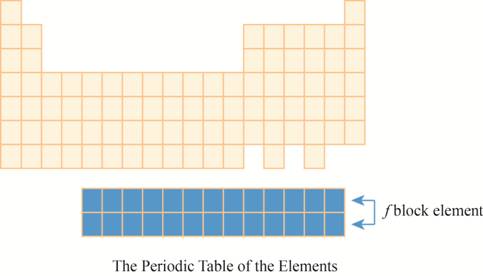
Figure 6
(g)
Interpretation:
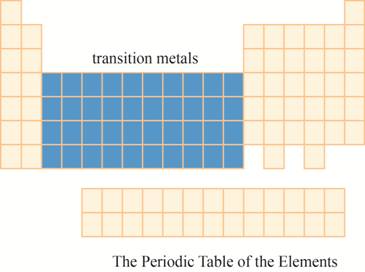
The region of transition metals on the periodic table is to be labeled.
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group.
Answer to Problem 33P
The transition metals are kept in ten columns in the middle of the main group elements on the periodic tableas shown below.
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
In the transition metals the
The region of transition metals on the periodic table is shown below.
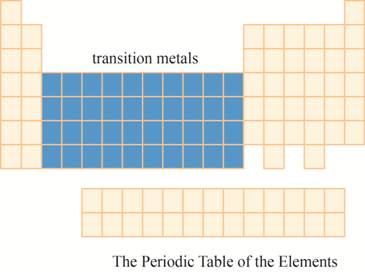
Figure 7
(h)
Interpretation:
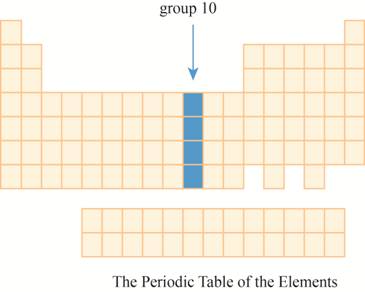
The region group
Concept Introduction:
The elements are placed in periodic table according to their atomic numbers. They are arranged in increasing order of atomic numbers systematically. The study of elements and their properties can be done easily as the elements with similar properties fall in the same group.
Answer to Problem 33P
This section has transition element.The region group
Explanation of Solution
The elements are organized on the basis of their increasing atomic number in periodic table.
These elements are organized in rows and columns. The rows are known as periods and columns as groups. Each period represents the outermost shell. As this outermost shell is filled the next atom goes to the next row or period.
The transition elements' inner shell gets filled. They have the electronic configuration of
The group
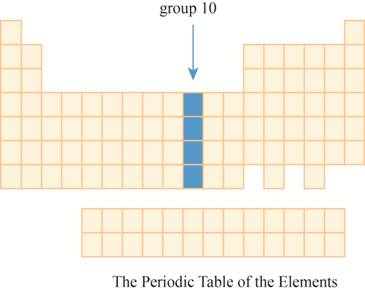
Figure 8
Want to see more full solutions like this?
Chapter 2 Solutions
General, Organic, and Biological Chemistry - 4th edition
Additional Science Textbook Solutions
Chemistry
EBK INTRODUCTION TO CHEMISTRY
Organic Chemistry
- Classify each of the following elements as a noble gas, representative element, transition element, or inner transition element. a. 1H b. 44Ru c. 51Sb d. 86Rnarrow_forwardFor each of the following sets of elements, label each as either noble gases, halogens, alkali metals, alkaline earth metals, or transition metals. a. Ti, Fe, Ag b. Mg, Sr, Ba c. Li, K., Rb d. Ne, Kr, Xe e. F, Br, Iarrow_forwardGive two examples of each: a. alkali metal b. alkaline earth metal c. halogen d. noble gas e. metal f. nonmetal g. transition metal h. metalloidarrow_forward
- Classify each of the following elements as a noble gas, representative element, transition element, or inner transition element. a. 15P b. 18Ar c. 79Au d. 92Uarrow_forwardScientists are trying to synthesize elements with more than 114 protons. State the expected atomic number of (a) the newest inert gas. (b) the new element with properties similar to those of the alkaline earth metals. (c) the new element that will behave like the halogens. (d) the new (nontransition) metal whose ion will have a +2 charge. (e) the new element that will start period 8.arrow_forwardWithout looking at your textbook or the periodic table, name three elements in each of the following groups (families). halogens alkali metals alkaline earth metals noble/inert gasesarrow_forward
- Using the periodic table, Identify the lightest member of each of the following groups: noble gases alkaline earth metals alkali metals chalcogensarrow_forwardWhich pair of elements do you expect to be most similar? a. Mg and Ca b. N and Cl c. Al and C d. S and Siarrow_forwardHow many elements exist with an atomic number greater than 20 that are a. Halogens b. Noble gases c. Alkali metals d. Alkaline earth metalsarrow_forward
- How many elements exist with an atomic number less than 40 that are a. Halogens b. Noble gases c. Alkali metals d. Alkaline earth metalsarrow_forwardSpecify the location of each of the following elements in the periodic table in terms of s area, p area, d area, or f area. a. Aluminum b. Potassium c. Sulfur d. Goldarrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





