
Concept explainers
Write a balanced chemical equation for the combustion of each of the following compounds:
a) Decane
b) Cyclodecane
c) Methylcyclononane
d) Cyclopentylcyclopentane
Interpretation:
A balanced chemical equation for the combustion of each of the given compounds is to be written.
Concept introduction:
Alkanes are inert in acid-base reactions but undergo oxidation-reduction reactions.
During combustion, alkanes undergo oxidation.
This combustion reaction of alkanes is exothermic, and the products formed are carbon dioxide and water.
When balancing the reaction, the carbon and hydrogen is balanced first, leaving oxygen for the last to balance.
Answer to Problem 39P
Solution:
a)
b)
c)
d)
Explanation of Solution
a) Decane
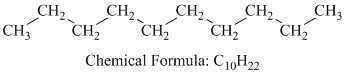
Decane is a straight chain alkane having the molecular formula
The combustion reaction of decane is written as follows:
To balance this reaction, first, the C and H are balanced.
Oxygen is balanced as follows:
The coefficients are converted to whole numbers as follows:
This is the complete balanced reaction of decane.
b) Cyclodecane
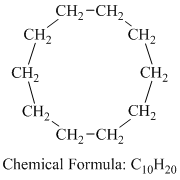
Cyclodecane is a cyclic alkane having the molecular formula
The combustion reaction of cyclodecane is written as follows:
To balance this reaction, first, the C and H are balanced.
Oxygen is balanced as follows:
This is the complete balanced reaction of cyclodecane.
c) Methylcyclononane
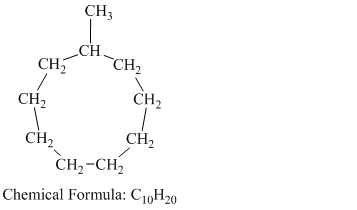
Methylcyclononane is a branched chain alkane having the molecular formula
The combustion reaction of methylcyclononane is written as follows:
To balance this reaction, first, the C and H are balanced.
Oxygen is balanced as follows:
This is the complete balanced reaction of methylcyclononane.
d) Cyclopentylcyclopentane
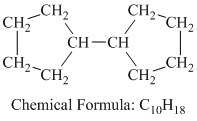
Cyclopentylcyclopentane is a branched chain alkane having the molecular formula
The combustion reaction of Cyclopentylcyclopentane is written as follows:
To balance this reaction, first, the C and H balanced.
Oxygen is balanced as follows:
The coefficients are converted to whole numbers as follows:
This is the complete balanced reaction of cyclopentylcyclopentane.
Want to see more full solutions like this?
Chapter 2 Solutions
ORGANIC CHEMISTRY (LL)-W/ACCESS
- Draw the structural diagram for each of the following compounds: a. cyclooctane b. methyl cyclopropanearrow_forwardPlease provide the structure and names of the isomers of the compound given. 1st isomer: has quarternary, secondary, and primary carbons 2nd isomer: has secondary and primary carbonsarrow_forwardProvide the IUPAC name. Indicate stereochemistry for stereocenters and alkene.arrow_forward
- Which has a higher melting point between tetracosane and eicosane? Explain.arrow_forwardGiven each of the IUPAC names provided, draw the corresponding structure. (a) 2-cyclopropoxypentane;(b) 1,2-dimethoxy-4-propylcyclohexane; (c) 4-(1,1-dimethylethyl)-1,2-dipropoxycyclooctanearrow_forwardWhat will be the color of the flame and the amount of soot if the following are ignited:(a) Hexane (b)Heptane(c) Cyclohexane (d) Cyclohexene (e) Benzene (f) Toluenearrow_forward
- Write the chemical equations involved for the reaction of cyclohexene with Br2arrow_forwardWrite a balanced equation for each of the following reactions. Show all possible products and write The IUPAC names for the products of the reactions. a. The complete combustion of cyclobutane b. The monobromination of propanearrow_forward1. Explain why 5,6-dichlorocyclohexene is an incorrect name for that compound. 2. Explain why 1,1-dimethylethene is an incorrect name for that compound.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


