
Concept explainers
Some Biochemical Reactions of
Alkanes occur naturally in places other than petroleum deposits—in insects, for example. The waxy alkanes dispersed in its cuticle help protect an insect from dehydration. Some insects use volatile alkanes to defend themselves or communicate with others of the same species. Alkanes even serve as starting materials that the insect converts to other biologically important substances.
The major biosynthetic pathway leading to alkanes is by enzyme-catalysed decarboxylation (loss of
Biochemical conversion of alkanes to other substances normally begins with oxidation.
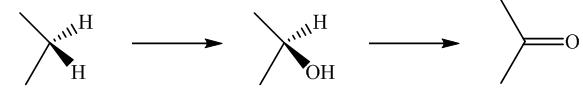
In addition to alkanes, the oxidation of drugs and other substances occurs mainly in the liver and is catalyzed by the enzyme cytochrome
Oxidation by microorganisms has been extensively studied and is often selective for certain kinds of

Tridecane
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry - Standalone book
- Explain the role strain plays on stability and reactivity of a cyclic alkane such as cyclopropane.arrow_forwardTopic: ALKANES INSTRUCTIONS: Place your answers in Microsoft Word Document (size: 8.5" x 11"). Show complete solutions for each item as needed.arrow_forward1. How will you carry out the following reactions? a. Combustion b. Bromination c. Oxidation2. Based on their chemical structures (both shape and atomic make-up), do you expect hydrocarbons (in general) to be more, or less, dense than water? Describe how it was done in the lab. 3. What colors are bromine and potassium permanganate solutions? 4. Name a substance/solution that could be used to distinguish alkane from an alkene. 5. Describe the general properties of hydrocarbons based on what you’ve learned in the module.arrow_forward
- 1. Give two organic compounds. The first compound must be a synthetic or man-made organic compound and the second compound must be a natural product or organic compound from natural sources (medicinal plant, coral, animal such as snail or microorganism such as bacteria or fungi). Compounds must have equal to or more than twenty carbon atoms. 2. Your chosen organic compound must have application in the management of human health or veterinary medicine. (for example for the treatment of pain or bacterial infection in human and animal).arrow_forwardThe skeletal line formula for a branched alkene is shown below. (i) What is the molecular formula of this compound? (ii) How many carbon atoms are in the longest chain, ignoring the double bond? (iii) What is the longest chain incorporating both carbons of the double bond? (iv) How many substituents are on this chain? (v) Give the IUPAC name for this compound. [6]arrow_forwardhow to name cyclic or acyclic alkanes ? what are the steps and rules that I have to follow to name anyone that comes my wayarrow_forward
- Natural gas and petroleum deposits make up the world’s largest source of alkanes. Refinement of petroleum followed by distillation produces fuels for vehicles where the fuels undergo combustion. However, not all fuels are judged as “good fuel”, some are “poor fuel”. Hence, the “octane number” was developed to determine the type of fuel. Why is the straight-chain alkane octane considered as poor fuel with an octane number = 0 and a branched-chain alkane 2,2,4-Trimethylpentane with an octane number = 100?arrow_forwardName and draw structural formulas for all alkenes with the molecular formula C5H10. As you draw these alkenes, remember that cis and trans isomers are different compounds and must be counted separatelyarrow_forwardPhysical State of Unbranched Hydrocarbons Procedure: In the activity sheet, ENCIRCLE the compound if it is a gas, UNDERLINE the compound if it is a liquid, and BOX the compound if it is solid at room temperature. Unbranched alkanes and unbranched alkenes or alkynes with only one or triple bond containing 1 to 4 carbon atoms (C1-C4) are GASES at room temperature. Unbranched alkanes and unbranched alkenes or alkynes with only one or triple bond containing 5 to 17 carbon atoms (C5-C17) are LIQUIDS at room temperature. Unbranched alkanes and unbranched alkenes or alkynes with only one or triple bond containing 18 or more carbon atoms (≥ C18) are SOLIDS at room temperature. CH3CH2CH3 – It is a GAS because it is an unbranched alkane containing 3 carbon atoms. 1-Hexene – It is a LIQUID because it is an unbranched alkene containing 6 carbon atoms (if you will illustrate the structure) with only one double bond. CH≡C-(CH2)16 -CH3 – It is a SOLID because it is an unbranched…arrow_forward
- Explain why alkenes are much more reactive than alkanes towards chlorine (CI2) or bromine (Br2) in the dark at room temperature, and why alkanes do not react with HCI (g) or HBr (g) whereas alkenes do.arrow_forwardIn this task you should explain structural isomerism in aliphatic alkanes and geometric isomerism in alkenes.arrow_forwardDraw the structures (a. expanded, b. condensed and c. skeletal structures) of the given hydrocarbon: an alkane with 7 carbon atoms a 5-carbon alkene with double bond on the 3rd carbon an alkyne with 6 carbon atoms, triple bond on the first carbon thank you for someone that will answer this!!arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




