
Concept explainers
Draw the products formed when each compound is treated with two equivalents of
a.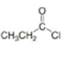 b.
b. 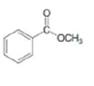 c.
c. 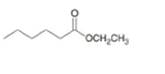
(a)
Interpretation: The product formed by the reaction of given compound with two equivalents of
Concept introduction: Grignard reagent is prepared by the reaction of alkyl or aryl bromide with magnesium metal in the presence of ether. The reaction of Grignard reagent with an aldehyde/ketone followed by hydrolysis yields an alcohol.
Answer to Problem 20.27P
The product formed by the reaction of given compound with two equivalents of
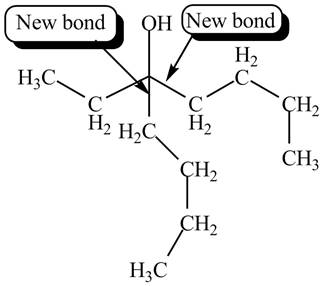
Explanation of Solution
The given compound is,
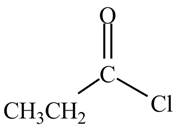
Figure 1
The product formed by the reaction of given compound with two equivalents of
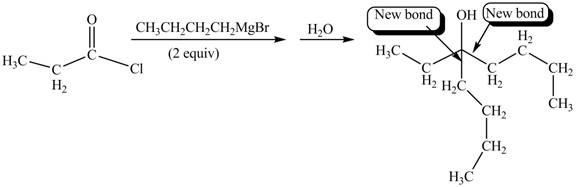
Figure 2
The first step involves the reaction of one equivalent of
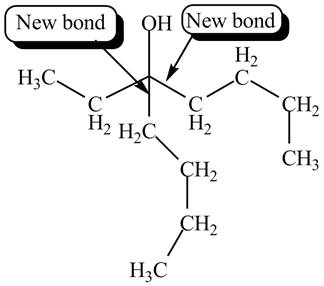
Figure 3
The product formed by the reaction of given compound with two equivalents of
(b)
Interpretation: The product formed by the reaction of given compound with two equivalents of
Concept introduction: Grignard reagent is prepared by the reaction of alkyl or aryl bromide with magnesium metal in the presence of ether. The reaction of Grignard reagent with an aldehyde/ketone followed by hydrolysis yields an alcohol.
Answer to Problem 20.27P
The product formed by the reaction of given compound with two equivalents of
Explanation of Solution
The given compound is,
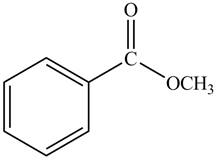
Figure 4
The product formed by the reaction of given compound with two equivalents of
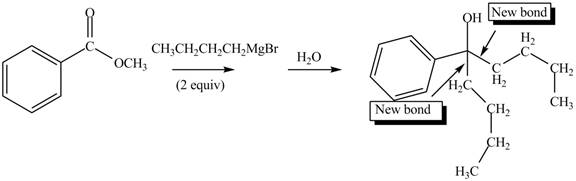
Figure 5
The first step involves the reaction of one equivalent of
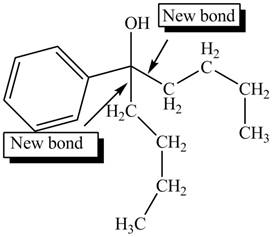
Figure 6
The product formed by the reaction of given compound with two equivalents of
(c)
Interpretation: The product formed by the reaction of given compound with two equivalents of
Concept introduction: Grignard reagent is prepared by the reaction of alkyl or aryl bromide with magnesium metal in the presence of ether. The reaction of Grignard reagent with an aldehyde/ketone followed by hydrolysis yields an alcohol.
Answer to Problem 20.27P
The product formed by the reaction of given compound with two equivalents of
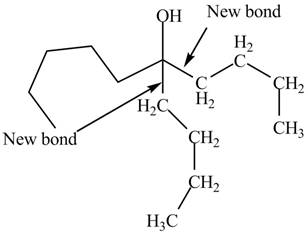
Explanation of Solution
The given compound is,
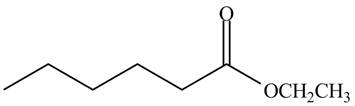
Figure 7
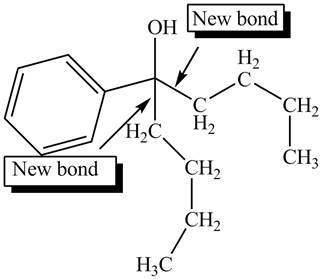
The product formed by the reaction of given compound with two equivalents of
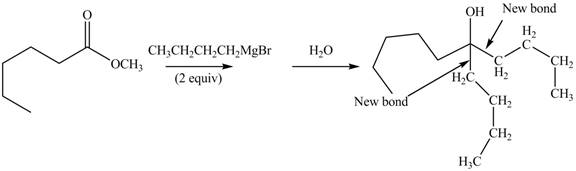
Figure 8
The first step involves the reaction of one equivalent of
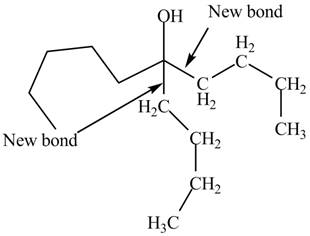
Figure 9
The product formed by the reaction of given compound with two equivalents of
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- Draw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forwardDraw the products formed from nitration of each compoundarrow_forward(a) Give an acceptable name for compound A. (b) Draw the organic products formed when A is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forward
- Draw the products formed when A or B is treated with each reagent. In some cases, no reaction occurs. [1] C6H5Li (excess); [2] H2Oarrow_forwardDraw the product formed when (CH3)2CHOH is treated with following reagent. H2SO4arrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. c. CH3CH2Cl, AlCl3 l. product in (c), then KMnO4arrow_forward
- Draw the hemiacetal and acetal formed when the carbonyl compound is treated with two equivalents of the given alcohol in the presence of H2SO4.arrow_forward(a) Give an acceptable name for each compound, (b) Draw the organic products formed when A or B is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forwardGive the IUPAC name for each aldehyde.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





