
Concept explainers
Draw the product formed when
a. 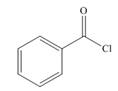 b.
b. 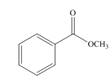 c.
c. 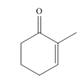 , then
, then
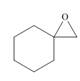 , then
, then
(a)
Interpretation: The product formed by the treatment of given compound with
Concept introduction: Organometallic reagents like
Answer to Problem 20.39P
The product formed by the treatment of given compound with
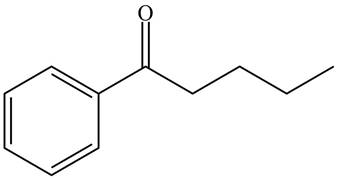
Figure 1
Explanation of Solution
Acid chlorides on reaction with organocuprate convert into ketone. The alkyl group of organocuprate occupies the position of leaving group
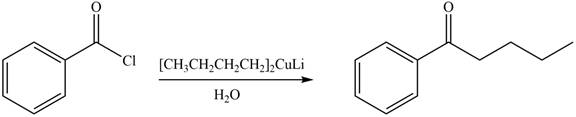
Figure 2
The product formed by the treatment of given compound with
(b)
Interpretation: The product formed by the treatment of given compound with
Concept introduction: Organometallic reagents like
Answer to Problem 20.39P
The given compound does not react with
Explanation of Solution
Esters on reaction with two equivalents of organometallic reagent convert into tertiary alcohol. However, it does not react with organocuprate because organocuprate are less reactive than
The given compound does not react with
(c)
Interpretation: The product formed by the treatment of given compound with
Concept introduction: Organometallic reagents like
Answer to Problem 20.39P
The product formed by the treatment of given compound with
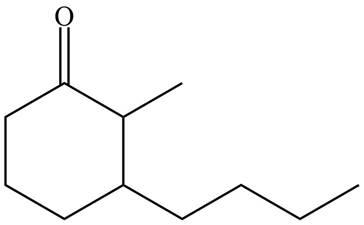
Figure 3
Explanation of Solution
Organocuprate reagent form
The product formed by the reaction of
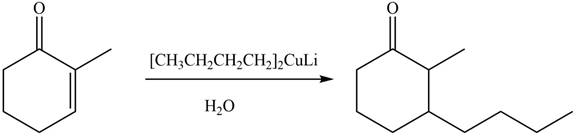
Figure 4
The product formed by the treatment of given compound with
(d)
Interpretation: The product formed by the treatment of given compound with
Concept introduction: Organometallic reagents like
Answer to Problem 20.39P
The product formed by the treatment of given compound with
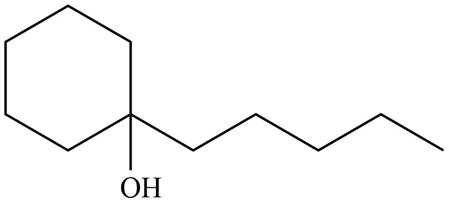
Figure 5
Explanation of Solution
Epoxides are obtained by treating alkene with m-chloroperbenzoic acid
The product formed by the treatment of given compound with
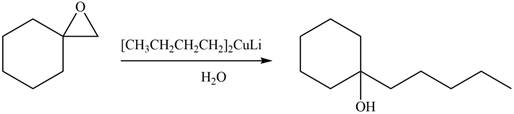
Figure 6
The product formed by the treatment of given compound with
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- Draw the product formed when (CH3)2CHOH is treated with each reagent. a.SOCl2, pyridine b. TsCl, pyridine c.H2SO4 d.HBr e.PBr3, then NaCN f.POCl3, pyridinearrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent. a. SOCl2, pyridine b. TsCl, pyridine c. H2SO4 d. HBr e. PBr3, then NaCN f. POCl3, pyridinearrow_forwardAnswer each question for A and B depicted in the ball-and-stick models.a. What is the IUPAC name for each compound?b. What product is formed when each compound is treated with NaOH?c. Name the products formed in part (b).d. Draw the structure of an isomer that is at least 105 times less acidic than each compound.arrow_forward
- Draw the products formed when A or B is treated with each reagent. In some cases, no reaction occurs. [1] C6H5Li (excess); [2] H2Oarrow_forward(a) Give an acceptable name for compound A. (b) Draw the organic products formed when A is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forwardWhat starting materials are needed to prepare each compound using a Heckreaction?arrow_forward
- Ethers are oxidized with O2 to form hydroperoxides that decompose violently when heated. Draw a stepwise mechanism for this reaction.arrow_forward(a) Give an acceptable name for each compound, (b) Draw the organic products formed when A or B is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forwardDraw the product formed when (CH3)2CHOH is treated with following reagent. HBrarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





