
Concept explainers
Interpretation:
Mechanism for biosynthetic steps involving 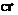 and water to explain how two cyclization steps could occur, is to be written.
and water to explain how two cyclization steps could occur, is to be written.
Concept introduction:
Electrophiles are electron deficient species that have positive or partially positive charge. Lewis acids are electrophiles that accept electron pair.
Nucleophiles are electron rich species that have negative or partially negative charge. Lewis bases are nucleophiles that donate electron pair.
Free radical is an atom, molecule, or ion that has an unpaired electron, which makes it highly chemically reactive.
Substitution reaction: A reaction in which one of the hydrogen atoms of a hydrocarbon or a functional group is substituted by any other functional group is called substitution reaction.
Elimination reaction: A reaction in which two substituent groups are detached and a double bond is formed is called elimination reaction.
Addition reaction: It is the reaction in which unsaturated bonds are converted to saturated molecules by the addition of molecules.
Vanadium complexes are present in water bodies and they are used to convert halide anions into cations in presence of hydrogen peroxides. These enzymes are known as halo peroxidases.
These vanadium complexes were discovered in the aquatic animals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Organic Chemistry
- Show how the synthetic scheme developed in Problem 23.67 can be modified to synthesize this triiodobenzoic acid X-ray contrast agent.arrow_forward(R)-Pulegone is converted to (R)-citronellic acid by addition of HCl followed by treatment with NaOH. Propose a mechanism for each step in this transformation and account for the regioselectivity of HCl addition.arrow_forwardShow how to convert carboxylic acids to other functional groups, and devisemultistep syntheses using carboxylic acids as starting materials and intermediates.Explain how acid chlorides are used as activated derivatives of carboxylic acidsarrow_forward
- Following is a retrosynthetic analysis for an intermediate in the industrial synthesis of vitamin A. (a) Addition of one mole of HCl to isoprene gives 4-chloro-2-methyl-2-butene as the major product. Propose a mechanism for this addition and account for its regioselectivity. (b) Propose a synthesis of the vitamin A precursor from this allylic chloride and ethyl acetoacetate.arrow_forwardThe pKa, of the conjugate acid of morpholine is 8.33. (a) Calculate the ratio of morpholine to morpholinium ion in aqueous solution at pH 7.0. (b) At what pH are the concentrations of morpholine and morpholinium ion equal?arrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forward
- One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (l) protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+. (a) Draw two resonance structures of diazomethane, and account for step 1. (b) What kind of reaction occurs in step 2?arrow_forwardThe formation of Br2 from NBS first involves the reaction of NBS with HBr to form an iminol intermediate and molecular bromine. The intermediate then undergoes acid-catalyzed tautomerism to form succinimide, the byproduct of the reaction. Propose a curved-arrow mechanism for the conversion of NBS into succinimide that also accounts for the formation of Br2.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT



