
PRACTICE PROBLEM 21.1
For each of the following complexes, determine the oxidation state of the metal and the total number of valence electrons it possesses.
(a)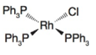
(b)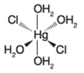
(c)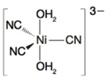
Interpretation:
The oxidation state of a metal and the total number of valence electrons in metals in the complexes is to be determined.
Concept introduction:
The oxidation state of a metal in a complex is the charge on the metal that would be there even if all the anionic ligands and counter ions were removed.
The total number of valence electrons of a metal in a complex is obtained by the following formula:
Total number of valence electrons of metal in complex =
Here,
Answer to Problem 1PP
Solution:
(a)
Oxidation state of Rh is
(b)
Oxidation state of Hg is
(c)
Oxidation state of Ni is
Explanation of Solution
Given information:
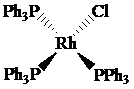
The oxidation state of rhodium is
Charge on rhodium is as follows:
Here,
Now, d-electrons in rhodium are
Total number of valence electrons of metal in complex =
Here,
For rhodium, the total number of valence electrons in the complex
Thus, the number of valence electrons in rhodium is
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Organic Chemistry
Chemistry
Chemistry: The Molecular Nature of Matter
Fundamentals of Heat and Mass Transfer
General, Organic, and Biological Chemistry (3rd Edition)
Chemistry For Changing Times (14th Edition)
- Please don't provide handwritten solution .... what is the written out electron pushing mechanism for thisarrow_forwardaminophylline is a good water soluble derivative of theophylline as ethylene diamine derivative in order to enhance its water solubility for formulation options and to enhance the bioavailability of theophylline . dose complexation phenomena involved in aminophylline preparation? how? show through drawing chemical structure as well as drawing the electronic configuration for complexationarrow_forwardCan ferrocene be acetylated twice at both iron atomsarrow_forward
- The following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Questions: (i) Give the name and suggest the colour of the precipitate B. Hence explain why it is necessary to recrystallize B several times. (ii) Why was it necessary to obtain a constant melting point for B?arrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. 1g of phenylamine yielded 1.2g of compound A. Calculate the percentage yield of the reaction.arrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Why was it necessary to obtain a constant melting point for B?arrow_forward
- The following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Give the structure of the product formed when an acidified solution of compound A is reacted with (i)Naphthalen-2-ol (2-naphthol) (ii) Sodium cyanidearrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Question: Answer both (i) and (ii) below (i) Give the structure of the product formed when an acidified solution of compound A is reacted with Naphthalen-2-ol (2-naphthol) and Sodium cyanide separately (ii) 1g of phenylamine yielded 1.2g of compound A. Calculate the percentage yield of the reaction.arrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. Write the equation for the reaction between compound A and phenol Give the name and suggest the colour of the precipitate B. Why is it necessary to recrystallize B several times?arrow_forward
- The following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. (a) What is the general name given to the reaction between phenylamine and sodium nitrite and explain why it's necessary to carry out the reaction at low temperatures. (b) Write the equation for the reaction between compound A and phenolarrow_forwardThe following is an experimental account of the preparation of compound B from Phenylamine. 2cm3 of phenylamine are dissolved in 10cm3 of 0.5M hydrochloric acid in a test tube and cooled in an ice bath. 5cm3 of 0.2M sodium nitrate solution cooled to about 5o are added to the solution above and shaken. A salt A, C6H5N2Cl is formed, collected and dried. 1g of salt A is dissolved in 10cm3 of water, cooled and added drop by drop to a cold solution of 0.3g phenol in 5cm3 of 0.1M sodium hydroxide solution. B is precipitated, filtered, dried and the melting point determined. B is further crystallized twice and the melting point taken each time and found to be constant. What is the general name given to the reaction between phenylamine and sodium nitrite and explain why it's necessary to carry out the reaction at low temperatures? Write the equation for the reaction between compound A and phenol Write the equation for the reaction between phenylamine and sodium nitrite.arrow_forwarddraw the electronic configuration of platinium IIII in ormaplatine (complex and expect the followings: a. the charge of this drug b. the shape of its complex. c. the expected type of hybridization d. the number of coordination covalent bonds in this drug e. what is the pharmacological use of ormaplatinePlease explain the point (d) and how I can calculate itarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





